articaine-hydrochloride
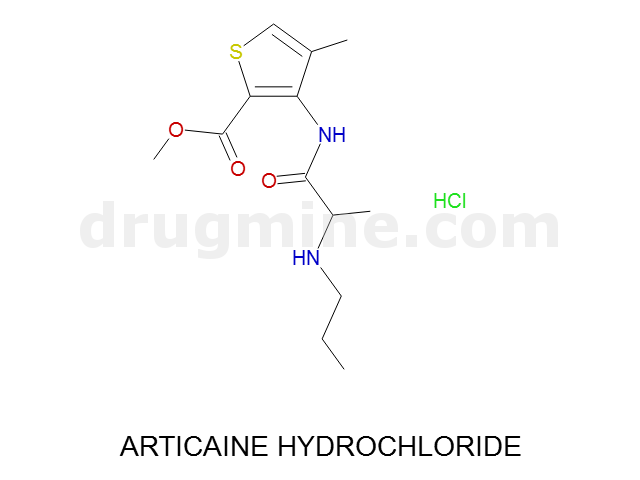

Name: ARTICAINE HYDROCHLORIDE
ID :
MW: 284
Number of atoms: 19
Molecular_Formula: C13H20N2O3S
Alogp: 2.072
Indication class : .
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
articaine hydrochloride containing products summary
There are in total 5 different products containing the active ingredient articaine hydrochloride. From the 5 drug products, zero have been discontinued.Product id = 10766
Application Number = 20971
Date of Application = 3,, Apr, 2000
RX/OTC/DISCN = RX
Tradename = SEPTOCAINE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = DEPROCO
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 4%;EQ 0.017MG BASE/1.7ML (4%;EQ 0.01MG BASE/ML)
----
Product id = 10765
Application Number = 22010
Date of Application = 30,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = SEPTOCAINE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = DEPROCO
ProductNo = 001
Tecode =
Rld = Yes
Strength = 4%;EQ 0.0085MG BASE/1.7ML (4%;EQ 0.005MG BASE/ML)
----
Product id = 9891
Application Number = 22466
Date of Application = 26,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = ORABLOC
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PIERREL
ProductNo = 001
Tecode =
Rld = No
Strength = 4%;EQ 0.009MG BASE/1.8ML (EQ 0.005MG BASE/ML)
----
Product id = 9892
Application Number = 22466
Date of Application = 26,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = ORABLOC
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PIERREL
ProductNo = 002
Tecode =
Rld = Yes
Strength = 4%;EQ 0.018MG BASE/1.8ML (EQ 0.01MG BASE/ML)
----
Product id = 6013
Application Number = 79138
Date of Application = 18,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = ARTICAINE HYDROCHLORIDE AND EPINEPHRINE BITARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 4%;EQ 0.017MG BASE/1.7ML (4%;EQ 0.01MG BASE/ML)
----