atenolol
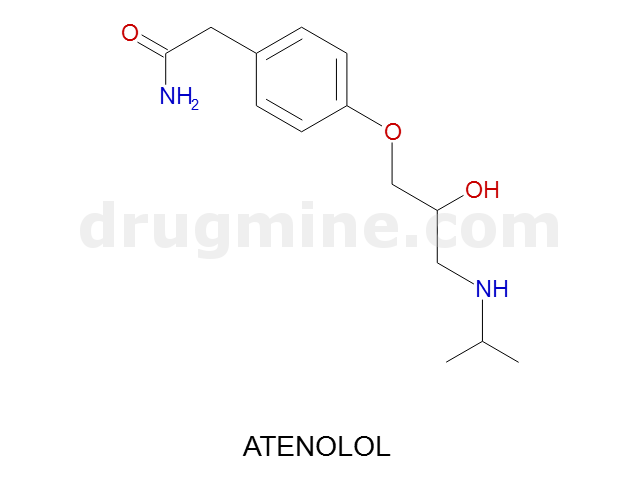

Name: ATENOLOL
ID :
MW: 266
Number of atoms: 19
Molecular_Formula: C14H22N2O3
Alogp: 0.669
Indication class : Anti-Adrenergic (beta-receptor)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
atenolol containing products summary
There are in total 78 different products containing the active ingredient atenolol. From the 78 drug products, 29 have been discontinued.Product id = 28556
Application Number = 18240
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TENORMIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ALVOGEN IPCO SARL
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 28557
Application Number = 18240
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TENORMIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ALVOGEN IPCO SARL
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 100MG
----
Product id = 28555
Application Number = 18240
Date of Application = 9,, Apr, 1990
RX/OTC/DISCN = RX
Tradename = TENORMIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ALVOGEN IPCO SARL
ProductNo = 004
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 28553
Application Number = 18760
Date of Application = 8,, Jun, 1984
RX/OTC/DISCN = RX
Tradename = TENORETIC 100
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ALVOGEN IPCO SARL
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 100MG;25MG
----
Product id = 28554
Application Number = 18760
Date of Application = 8,, Jun, 1984
RX/OTC/DISCN = RX
Tradename = TENORETIC 50
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ALVOGEN IPCO SARL
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG;25MG
----
Product id = 10960
Application Number = 19058
Date of Application = 13,, Sep, 1989
RX/OTC/DISCN = DISCN
Tradename = TENORMIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.5MG/ML **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 18158
Application Number = 72301
Date of Application = 31,, May, 1990
RX/OTC/DISCN = RX
Tradename = ATENOLOL AND CHLORTHALIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG;25MG
----
Product id = 18159
Application Number = 72302
Date of Application = 31,, May, 1990
RX/OTC/DISCN = RX
Tradename = ATENOLOL AND CHLORTHALIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG;25MG
----
Product id = 18113
Application Number = 72303
Date of Application = 15,, Jul, 1988
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18114
Application Number = 72304
Date of Application = 15,, Jul, 1988
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18134
Application Number = 73025
Date of Application = 17,, Sep, 1991
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18136
Application Number = 73026
Date of Application = 17,, Sep, 1991
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18144
Application Number = 73315
Date of Application = 28,, May, 1993
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18146
Application Number = 73316
Date of Application = 28,, May, 1993
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18101
Application Number = 73317
Date of Application = 20,, Mar, 1992
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18102
Application Number = 73318
Date of Application = 20,, Mar, 1992
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18153
Application Number = 73352
Date of Application = 27,, Dec, 1991
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18154
Application Number = 73353
Date of Application = 27,, Dec, 1991
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18122
Application Number = 73457
Date of Application = 24,, Jan, 1992
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18118
Application Number = 73457
Date of Application = 26,, Apr, 1999
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18120
Application Number = 73457
Date of Application = 24,, Jan, 1992
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18116
Application Number = 73475
Date of Application = 30,, Mar, 1993
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18117
Application Number = 73476
Date of Application = 30,, Mar, 1993
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18107
Application Number = 73542
Date of Application = 19,, Dec, 1991
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18108
Application Number = 73543
Date of Application = 19,, Dec, 1991
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18160
Application Number = 73581
Date of Application = 29,, Apr, 1993
RX/OTC/DISCN = RX
Tradename = ATENOLOL AND CHLORTHALIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG;25MG
----
Product id = 18161
Application Number = 73582
Date of Application = 29,, Apr, 1993
RX/OTC/DISCN = RX
Tradename = ATENOLOL AND CHLORTHALIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG;25MG
----
Product id = 18112
Application Number = 73646
Date of Application = 31,, Jul, 1992
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18168
Application Number = 73665
Date of Application = 2,, Jul, 1992
RX/OTC/DISCN = RX
Tradename = ATENOLOL AND CHLORTHALIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG;25MG
----
Product id = 18169
Application Number = 73665
Date of Application = 2,, Jul, 1992
RX/OTC/DISCN = RX
Tradename = ATENOLOL AND CHLORTHALIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG;25MG
----
Product id = 18138
Application Number = 73676
Date of Application = 30,, Oct, 1992
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SCS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18139
Application Number = 73676
Date of Application = 30,, Oct, 1992
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SCS
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18132
Application Number = 74052
Date of Application = 1,, May, 1992
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18145
Application Number = 74056
Date of Application = 18,, Jan, 1995
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18147
Application Number = 74056
Date of Application = 18,, Jan, 1995
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18143
Application Number = 74056
Date of Application = 19,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18106
Application Number = 74099
Date of Application = 28,, Apr, 1992
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18129
Application Number = 74101
Date of Application = 17,, Jul, 1997
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18130
Application Number = 74101
Date of Application = 17,, Jul, 1997
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18131
Application Number = 74101
Date of Application = 17,, Jul, 1997
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18166
Application Number = 74107
Date of Application = 24,, Sep, 1997
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL AND CHLORTHALIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;25MG
----
Product id = 18167
Application Number = 74107
Date of Application = 24,, Sep, 1997
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL AND CHLORTHALIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG;25MG
----
Product id = 18148
Application Number = 74120
Date of Application = 24,, Feb, 1995
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18149
Application Number = 74120
Date of Application = 24,, Feb, 1995
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18121
Application Number = 74126
Date of Application = 23,, Mar, 1994
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18123
Application Number = 74126
Date of Application = 23,, Mar, 1994
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18119
Application Number = 74126
Date of Application = 26,, Aug, 1998
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18127
Application Number = 74127
Date of Application = 21,, Feb, 1995
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NOSTRUM LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18128
Application Number = 74127
Date of Application = 21,, Feb, 1995
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NOSTRUM LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18162
Application Number = 74203
Date of Application = 31,, Oct, 1993
RX/OTC/DISCN = RX
Tradename = ATENOLOL AND CHLORTHALIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG;25MG
----
Product id = 18163
Application Number = 74203
Date of Application = 31,, Oct, 1993
RX/OTC/DISCN = RX
Tradename = ATENOLOL AND CHLORTHALIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG;25MG
----
Product id = 18133
Application Number = 74265
Date of Application = 28,, Feb, 1994
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18135
Application Number = 74265
Date of Application = 28,, Feb, 1994
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18137
Application Number = 74265
Date of Application = 28,, Feb, 1994
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18164
Application Number = 74404
Date of Application = 14,, May, 1998
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL AND CHLORTHALIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NOSTRUM LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;25MG
----
Product id = 18165
Application Number = 74404
Date of Application = 14,, May, 1998
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL AND CHLORTHALIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NOSTRUM LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG;25MG
----
Product id = 18115
Application Number = 74499
Date of Application = 30,, Jul, 1997
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18155
Application Number = 76900
Date of Application = 28,, Jan, 2005
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18156
Application Number = 76900
Date of Application = 28,, Jan, 2005
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18157
Application Number = 76900
Date of Application = 28,, Jan, 2005
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18098
Application Number = 76907
Date of Application = 30,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABLE
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18099
Application Number = 76907
Date of Application = 30,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABLE
ProductNo = 002
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18100
Application Number = 76907
Date of Application = 30,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABLE
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18150
Application Number = 77443
Date of Application = 13,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNIQUE PHARM LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18151
Application Number = 77443
Date of Application = 13,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNIQUE PHARM LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18152
Application Number = 77443
Date of Application = 13,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNIQUE PHARM LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18109
Application Number = 77877
Date of Application = 27,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18110
Application Number = 77877
Date of Application = 27,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18111
Application Number = 77877
Date of Application = 27,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IPCA LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18140
Application Number = 78210
Date of Application = 10,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18141
Application Number = 78210
Date of Application = 10,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18142
Application Number = 78210
Date of Application = 10,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18124
Application Number = 78254
Date of Application = 25,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NORTHSTAR HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18125
Application Number = 78254
Date of Application = 25,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NORTHSTAR HLTHCARE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18126
Application Number = 78254
Date of Application = 25,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NORTHSTAR HLTHCARE
ProductNo = 003
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18103
Application Number = 78512
Date of Application = 31,, Oct, 2007
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18104
Application Number = 78512
Date of Application = 31,, Oct, 2007
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18105
Application Number = 78512
Date of Application = 31,, Oct, 2007
RX/OTC/DISCN = RX
Tradename = ATENOLOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 100MG
----