benazepril-hydrochloride
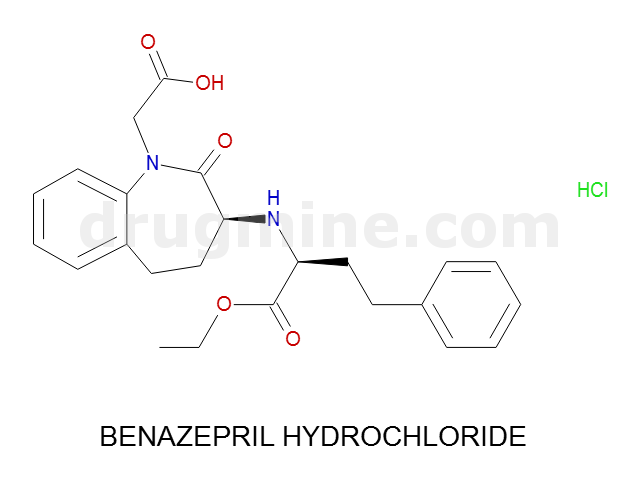


Name: BENAZEPRIL HYDROCHLORIDE
ID :
MW: 424
Number of atoms: 31
Molecular_Formula: C24H28N2O5
Alogp: 0.621
Indication class : Enzyme Inhibitor (angiotensin-converting)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
benazepril hydrochloride containing products summary
There are in total 137 different products containing the active ingredient benazepril hydrochloride. From the 137 drug products, 8 have been discontinued.Product id = 24038
Application Number = 19851
Date of Application = 25,, Jun, 1991
RX/OTC/DISCN = RX
Tradename = LOTENSIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 24039
Application Number = 19851
Date of Application = 25,, Jun, 1991
RX/OTC/DISCN = RX
Tradename = LOTENSIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 24040
Application Number = 19851
Date of Application = 25,, Jun, 1991
RX/OTC/DISCN = RX
Tradename = LOTENSIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 24041
Application Number = 19851
Date of Application = 25,, Jun, 1991
RX/OTC/DISCN = RX
Tradename = LOTENSIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 004
Tecode = AB
Rld = Yes
Strength = 40MG
----
Product id = 24042
Application Number = 20033
Date of Application = 19,, May, 1992
RX/OTC/DISCN = RX
Tradename = LOTENSIN HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 24043
Application Number = 20033
Date of Application = 19,, May, 1992
RX/OTC/DISCN = RX
Tradename = LOTENSIN HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 24045
Application Number = 20033
Date of Application = 19,, May, 1992
RX/OTC/DISCN = RX
Tradename = LOTENSIN HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 20MG;25MG
----
Product id = 24044
Application Number = 20033
Date of Application = 19,, May, 1992
RX/OTC/DISCN = RX
Tradename = LOTENSIN HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = US PHARMS HOLDINGS I
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 2403
Application Number = 20364
Date of Application = 3,, Mar, 1995
RX/OTC/DISCN = RX
Tradename = LOTREL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 2.5MG BASE;10MG
----
Product id = 2404
Application Number = 20364
Date of Application = 3,, Mar, 1995
RX/OTC/DISCN = RX
Tradename = LOTREL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;10MG
----
Product id = 2405
Application Number = 20364
Date of Application = 3,, Mar, 1995
RX/OTC/DISCN = RX
Tradename = LOTREL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;20MG
----
Product id = 2407
Application Number = 20364
Date of Application = 20,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = LOTREL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;20MG
----
Product id = 2408
Application Number = 20364
Date of Application = 11,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = LOTREL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 006
Tecode = AB
Rld = Yes
Strength = EQ 10MG BASE;40MG
----
Product id = 2406
Application Number = 20364
Date of Application = 11,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = LOTREL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 007
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;40MG
----
Product id = 18366
Application Number = 76118
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18367
Application Number = 76118
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18368
Application Number = 76118
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18369
Application Number = 76118
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 18378
Application Number = 76211
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18379
Application Number = 76211
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18380
Application Number = 76211
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18381
Application Number = 76211
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 004
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 18339
Application Number = 76267
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18340
Application Number = 76267
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18341
Application Number = 76267
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18342
Application Number = 76267
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 18358
Application Number = 76333
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18359
Application Number = 76333
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18360
Application Number = 76333
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18361
Application Number = 76333
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 18386
Application Number = 76342
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18387
Application Number = 76342
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 18388
Application Number = 76342
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 18389
Application Number = 76342
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG;25MG
----
Product id = 18370
Application Number = 76344
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18371
Application Number = 76344
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18372
Application Number = 76344
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18373
Application Number = 76344
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 004
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 18394
Application Number = 76348
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18395
Application Number = 76348
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 18396
Application Number = 76348
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 18397
Application Number = 76348
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG;25MG
----
Product id = 18374
Application Number = 76402
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18375
Application Number = 76402
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18376
Application Number = 76402
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18377
Application Number = 76402
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 18362
Application Number = 76430
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18363
Application Number = 76430
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18364
Application Number = 76430
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18365
Application Number = 76430
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 18354
Application Number = 76476
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = DISCN
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GENPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 18355
Application Number = 76476
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = DISCN
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GENPHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 18356
Application Number = 76476
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = DISCN
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GENPHARM
ProductNo = 003
Tecode =
Rld = No
Strength = 20MG
----
Product id = 18357
Application Number = 76476
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = DISCN
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GENPHARM
ProductNo = 004
Tecode =
Rld = No
Strength = 40MG
----
Product id = 18402
Application Number = 76612
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = DISCN
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18403
Application Number = 76612
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = DISCN
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG;12.5MG
----
Product id = 18404
Application Number = 76612
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = DISCN
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode =
Rld = No
Strength = 20MG;12.5MG
----
Product id = 18405
Application Number = 76612
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = DISCN
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 004
Tecode =
Rld = No
Strength = 20MG;25MG
----
Product id = 18410
Application Number = 76631
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18411
Application Number = 76631
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 18412
Application Number = 76631
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 18413
Application Number = 76631
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG;25MG
----
Product id = 18398
Application Number = 76688
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18399
Application Number = 76688
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 18400
Application Number = 76688
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 18401
Application Number = 76688
Date of Application = 11,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG;25MG
----
Product id = 18343
Application Number = 76820
Date of Application = 3,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18344
Application Number = 76820
Date of Application = 3,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS LLC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18345
Application Number = 76820
Date of Application = 3,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS LLC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18346
Application Number = 76820
Date of Application = 3,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS LLC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 18347
Application Number = 77128
Date of Application = 8,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18348
Application Number = 77128
Date of Application = 8,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18349
Application Number = 77128
Date of Application = 8,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18350
Application Number = 77128
Date of Application = 8,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 1003
Application Number = 77179
Date of Application = 18,, May, 2007
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2.5MG BASE;10MG
----
Product id = 1004
Application Number = 77179
Date of Application = 18,, May, 2007
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;10MG
----
Product id = 1005
Application Number = 77179
Date of Application = 18,, May, 2007
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;20MG
----
Product id = 1007
Application Number = 77179
Date of Application = 18,, May, 2007
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;20MG
----
Product id = 1006
Application Number = 77179
Date of Application = 5,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;40MG
----
Product id = 1008
Application Number = 77179
Date of Application = 5,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;40MG
----
Product id = 979
Application Number = 77183
Date of Application = 15,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2.5MG BASE;10MG
----
Product id = 980
Application Number = 77183
Date of Application = 15,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;10MG
----
Product id = 981
Application Number = 77183
Date of Application = 15,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;20MG
----
Product id = 983
Application Number = 77183
Date of Application = 15,, Apr, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;20MG
----
Product id = 991
Application Number = 77375
Date of Application = 21,, May, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2.5MG BASE;10MG
----
Product id = 992
Application Number = 77375
Date of Application = 21,, May, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;10MG
----
Product id = 993
Application Number = 77375
Date of Application = 21,, May, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;20MG
----
Product id = 995
Application Number = 77375
Date of Application = 21,, May, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;20MG
----
Product id = 18406
Application Number = 77483
Date of Application = 8,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18407
Application Number = 77483
Date of Application = 8,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 18408
Application Number = 77483
Date of Application = 8,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 18409
Application Number = 77483
Date of Application = 8,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG;25MG
----
Product id = 1009
Application Number = 77890
Date of Application = 14,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2.5MG BASE;10MG
----
Product id = 1010
Application Number = 77890
Date of Application = 14,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;10MG
----
Product id = 1011
Application Number = 77890
Date of Application = 14,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;20MG
----
Product id = 1012
Application Number = 77890
Date of Application = 14,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;20MG
----
Product id = 18351
Application Number = 78212
Date of Application = 22,, May, 2008
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18352
Application Number = 78212
Date of Application = 22,, May, 2008
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18353
Application Number = 78212
Date of Application = 22,, May, 2008
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 997
Application Number = 78381
Date of Application = 29,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2.5MG BASE;10MG
----
Product id = 998
Application Number = 78381
Date of Application = 29,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;10MG
----
Product id = 999
Application Number = 78381
Date of Application = 29,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;20MG
----
Product id = 1001
Application Number = 78381
Date of Application = 29,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;20MG
----
Product id = 1000
Application Number = 78381
Date of Application = 29,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;40MG
----
Product id = 1002
Application Number = 78381
Date of Application = 29,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;40MG
----
Product id = 985
Application Number = 78466
Date of Application = 5,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LUPIN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2.5MG BASE;10MG
----
Product id = 986
Application Number = 78466
Date of Application = 5,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LUPIN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;10MG
----
Product id = 987
Application Number = 78466
Date of Application = 5,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LUPIN PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;20MG
----
Product id = 989
Application Number = 78466
Date of Application = 5,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LUPIN PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;20MG
----
Product id = 988
Application Number = 78466
Date of Application = 5,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LUPIN PHARMS
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;40MG
----
Product id = 990
Application Number = 78466
Date of Application = 5,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LUPIN PHARMS
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;40MG
----
Product id = 18390
Application Number = 78794
Date of Application = 21,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18391
Application Number = 78794
Date of Application = 21,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 18392
Application Number = 78794
Date of Application = 21,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 18393
Application Number = 78794
Date of Application = 21,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 20MG;25MG
----
Product id = 18382
Application Number = 78848
Date of Application = 23,, May, 2008
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18383
Application Number = 78848
Date of Application = 23,, May, 2008
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18384
Application Number = 78848
Date of Application = 23,, May, 2008
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 18385
Application Number = 78848
Date of Application = 23,, May, 2008
RX/OTC/DISCN = RX
Tradename = BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 004
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 994
Application Number = 79047
Date of Application = 5,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;40MG
----
Product id = 996
Application Number = 79047
Date of Application = 5,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;40MG
----
Product id = 982
Application Number = 90149
Date of Application = 5,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;40MG
----
Product id = 984
Application Number = 90149
Date of Application = 5,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;40MG
----
Product id = 1013
Application Number = 90364
Date of Application = 5,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;40MG
----
Product id = 1014
Application Number = 90364
Date of Application = 5,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;40MG
----
Product id = 967
Application Number = 91431
Date of Application = 30,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2.5MG BASE;10MG
----
Product id = 968
Application Number = 91431
Date of Application = 30,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;10MG
----
Product id = 969
Application Number = 91431
Date of Application = 30,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;20MG
----
Product id = 970
Application Number = 91431
Date of Application = 30,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;40MG
----
Product id = 971
Application Number = 91431
Date of Application = 30,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;20MG
----
Product id = 972
Application Number = 91431
Date of Application = 30,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;40MG
----
Product id = 973
Application Number = 202239
Date of Application = 5,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2.5MG BASE;10MG
----
Product id = 974
Application Number = 202239
Date of Application = 5,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;10MG
----
Product id = 975
Application Number = 202239
Date of Application = 5,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;20MG
----
Product id = 976
Application Number = 202239
Date of Application = 5,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;40MG
----
Product id = 977
Application Number = 202239
Date of Application = 5,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;20MG
----
Product id = 978
Application Number = 202239
Date of Application = 5,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND BENAZEPRIL HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;40MG
----