butorphanol-tartrate
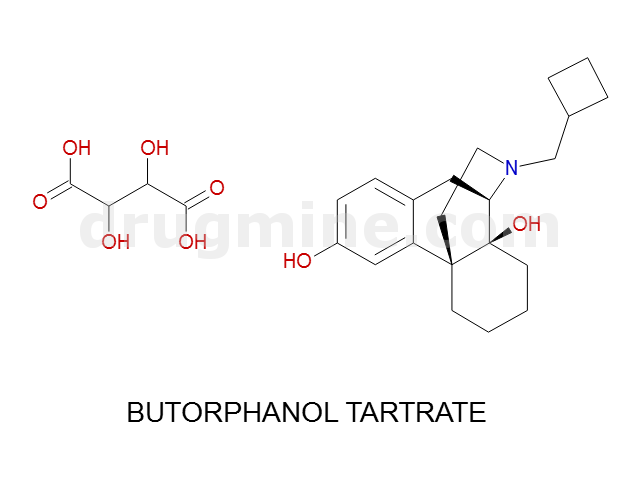

Name: BUTORPHANOL TARTRATE
ID :
MW: 327
Number of atoms: 24
Molecular_Formula: C21H29NO2
Alogp: 3.846
Indication class : Analgesic; Antitussive
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
butorphanol tartrate containing products summary
There are in total 26 different products containing the active ingredient butorphanol tartrate. From the 26 drug products, 16 have been discontinued.Product id = 10867
Application Number = 17857
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = STADOL PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 10868
Application Number = 17857
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = STADOL PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = 2MG/ML
----
Product id = 10866
Application Number = 17857
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = STADOL
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = APOTHECON
ProductNo = 004
Tecode =
Rld = No
Strength = 2MG/ML
----
Product id = 14396
Application Number = 19890
Date of Application = 12,, Dec, 1991
RX/OTC/DISCN = DISCN
Tradename = STADOL
Route/format = NASAL / SPRAY, METERED
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/SPRAY
----
Product id = 6233
Application Number = 74620
Date of Application = 22,, Jan, 1997
RX/OTC/DISCN = DISCN
Tradename = BUTORPHANOL TARTRATE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 6236
Application Number = 74620
Date of Application = 22,, Jan, 1997
RX/OTC/DISCN = DISCN
Tradename = BUTORPHANOL TARTRATE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode =
Rld = No
Strength = 2MG/ML
----
Product id = 6234
Application Number = 74626
Date of Application = 23,, Jan, 1997
RX/OTC/DISCN = RX
Tradename = BUTORPHANOL TARTRATE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 6237
Application Number = 74626
Date of Application = 23,, Jan, 1997
RX/OTC/DISCN = RX
Tradename = BUTORPHANOL TARTRATE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 2MG/ML
----
Product id = 6231
Application Number = 75045
Date of Application = 12,, Aug, 1998
RX/OTC/DISCN = RX
Tradename = BUTORPHANOL TARTRATE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 6232
Application Number = 75045
Date of Application = 12,, Aug, 1998
RX/OTC/DISCN = RX
Tradename = BUTORPHANOL TARTRATE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 002
Tecode = AP
Rld = No
Strength = 2MG/ML
----
Product id = 6221
Application Number = 75046
Date of Application = 12,, Aug, 1998
RX/OTC/DISCN = RX
Tradename = BUTORPHANOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 2MG/ML
----
Product id = 6235
Application Number = 75170
Date of Application = 28,, Sep, 1998
RX/OTC/DISCN = DISCN
Tradename = BUTORPHANOL TARTRATE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 6238
Application Number = 75170
Date of Application = 28,, Sep, 1998
RX/OTC/DISCN = DISCN
Tradename = BUTORPHANOL TARTRATE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode =
Rld = No
Strength = 2MG/ML
----
Product id = 6225
Application Number = 75342
Date of Application = 4,, Nov, 1999
RX/OTC/DISCN = DISCN
Tradename = BUTORPHANOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 6227
Application Number = 75342
Date of Application = 4,, Nov, 1999
RX/OTC/DISCN = DISCN
Tradename = BUTORPHANOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode =
Rld = No
Strength = 2MG/ML
----
Product id = 14344
Application Number = 75499
Date of Application = 4,, Dec, 2002
RX/OTC/DISCN = RX
Tradename = BUTORPHANOL TARTRATE
Route/format = NASAL / SPRAY, METERED
Application Type = A
Applicant Name = NOVEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG/SPRAY
----
Product id = 6226
Application Number = 75559
Date of Application = 20,, Mar, 2000
RX/OTC/DISCN = DISCN
Tradename = BUTORPHANOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 6228
Application Number = 75559
Date of Application = 20,, Mar, 2000
RX/OTC/DISCN = DISCN
Tradename = BUTORPHANOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode =
Rld = No
Strength = 2MG/ML
----
Product id = 6229
Application Number = 75695
Date of Application = 23,, Oct, 2001
RX/OTC/DISCN = DISCN
Tradename = BUTORPHANOL TARTRATE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 6230
Application Number = 75695
Date of Application = 23,, Oct, 2001
RX/OTC/DISCN = DISCN
Tradename = BUTORPHANOL TARTRATE PRESERVATIVE FREE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode =
Rld = No
Strength = 2MG/ML
----
Product id = 6220
Application Number = 75697
Date of Application = 23,, Oct, 2001
RX/OTC/DISCN = DISCN
Tradename = BUTORPHANOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG/ML
----
Product id = 14343
Application Number = 75759
Date of Application = 8,, Aug, 2001
RX/OTC/DISCN = RX
Tradename = BUTORPHANOL TARTRATE
Route/format = NASAL / SPRAY, METERED
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 1MG/SPRAY
----
Product id = 14345
Application Number = 75824
Date of Application = 12,, Mar, 2002
RX/OTC/DISCN = RX
Tradename = BUTORPHANOL TARTRATE
Route/format = NASAL / SPRAY, METERED
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 1MG/SPRAY
----
Product id = 6223
Application Number = 78247
Date of Application = 29,, Apr, 2009
RX/OTC/DISCN = DISCN
Tradename = BUTORPHANOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG/ML
----
Product id = 6222
Application Number = 78400
Date of Application = 1,, May, 2009
RX/OTC/DISCN = RX
Tradename = BUTORPHANOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1MG/ML
----
Product id = 6224
Application Number = 78400
Date of Application = 1,, May, 2009
RX/OTC/DISCN = RX
Tradename = BUTORPHANOL TARTRATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 002
Tecode = AP
Rld = No
Strength = 2MG/ML
----