candesartan-cilexetil
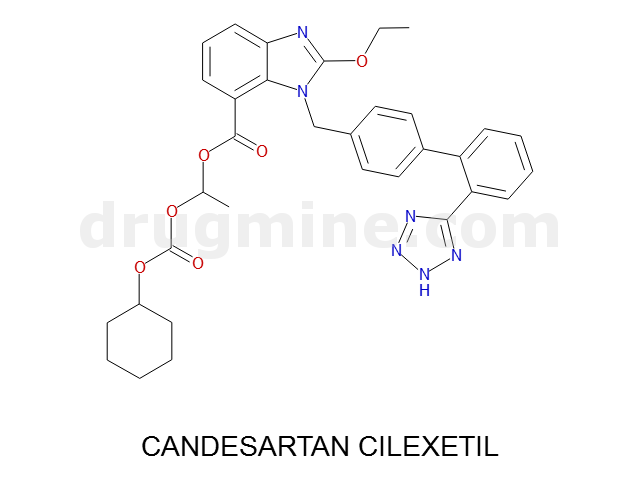

Name: CANDESARTAN CILEXETIL
ID :
MW: 611
Number of atoms: 45
Molecular_Formula: C33H34N6O6
Alogp: 7.353
Indication class : Antagonist (angiotensin II receptor); Antihypertensive
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
candesartan cilexetil containing products summary
There are in total 27 different products containing the active ingredient candesartan cilexetil. From the 27 drug products, zero have been discontinued.Product id = 18087
Application Number = 20838
Date of Application = 4,, Jun, 1998
RX/OTC/DISCN = RX
Tradename = ATACAND
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 18088
Application Number = 20838
Date of Application = 4,, Jun, 1998
RX/OTC/DISCN = RX
Tradename = ATACAND
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 8MG
----
Product id = 18089
Application Number = 20838
Date of Application = 4,, Jun, 1998
RX/OTC/DISCN = RX
Tradename = ATACAND
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 16MG
----
Product id = 18090
Application Number = 20838
Date of Application = 4,, Jun, 1998
RX/OTC/DISCN = RX
Tradename = ATACAND
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 004
Tecode = AB
Rld = Yes
Strength = 32MG
----
Product id = 18091
Application Number = 21093
Date of Application = 5,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = ATACAND HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 16MG;12.5MG
----
Product id = 18092
Application Number = 21093
Date of Application = 5,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = ATACAND HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 32MG;12.5MG
----
Product id = 18093
Application Number = 21093
Date of Application = 16,, May, 2008
RX/OTC/DISCN = RX
Tradename = ATACAND HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ASTRAZENECA
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 32MG;25MG
----
Product id = 18806
Application Number = 78702
Date of Application = 3,, May, 2013
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 18807
Application Number = 78702
Date of Application = 3,, May, 2013
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 8MG
----
Product id = 18808
Application Number = 78702
Date of Application = 3,, May, 2013
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 16MG
----
Product id = 18809
Application Number = 78702
Date of Application = 3,, May, 2013
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode = AB
Rld = No
Strength = 32MG
----
Product id = 18819
Application Number = 90704
Date of Application = 4,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 16MG;12.5MG
----
Product id = 18820
Application Number = 90704
Date of Application = 4,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 32MG;12.5MG
----
Product id = 18821
Application Number = 90704
Date of Application = 4,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 32MG;25MG
----
Product id = 18802
Application Number = 202079
Date of Application = 10,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 18803
Application Number = 202079
Date of Application = 10,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 8MG
----
Product id = 18804
Application Number = 202079
Date of Application = 10,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 16MG
----
Product id = 18805
Application Number = 202079
Date of Application = 10,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 32MG
----
Product id = 18810
Application Number = 202884
Date of Application = 4,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 16MG;12.5MG
----
Product id = 18811
Application Number = 202884
Date of Application = 4,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 32MG;12.5MG
----
Product id = 18812
Application Number = 202884
Date of Application = 3,, Jun, 2013
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 32MG;25MG
----
Product id = 18813
Application Number = 202965
Date of Application = 3,, Jun, 2013
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 16MG;12.5MG
----
Product id = 18814
Application Number = 202965
Date of Application = 3,, Jun, 2013
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 32MG;12.5MG
----
Product id = 18815
Application Number = 202965
Date of Application = 3,, Jun, 2013
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 32MG;25MG
----
Product id = 18816
Application Number = 204100
Date of Application = 27,, Feb, 2015
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 16MG;12.5MG
----
Product id = 18817
Application Number = 204100
Date of Application = 27,, Feb, 2015
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 32MG;12.5MG
----
Product id = 18818
Application Number = 204100
Date of Application = 27,, Feb, 2015
RX/OTC/DISCN = RX
Tradename = CANDESARTAN CILEXETIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 32MG;25MG
----