captopril
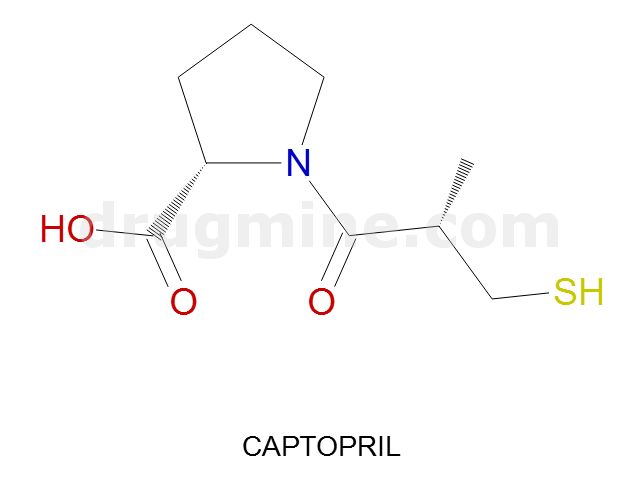


Name: CAPTOPRIL
ID :
MW: 217
Number of atoms: 14
Molecular_Formula: C9H15NO3S
Alogp: 0.667
Indication class : Enzyme Inhibitor (angiotensin-converting); Antihypertensive
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
captopril containing products summary
There are in total 120 different products containing the active ingredient captopril. From the 120 drug products, 76 have been discontinued.Product id = 18832
Application Number = 18343
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CAPOTEN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PAR PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18830
Application Number = 18343
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CAPOTEN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PAR PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18834
Application Number = 18343
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CAPOTEN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PAR PHARM
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 100MG
----
Product id = 18835
Application Number = 18343
Date of Application = 13,, Jun, 1995
RX/OTC/DISCN = DISCN
Tradename = CAPOTEN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PAR PHARM
ProductNo = 004
Tecode =
Rld = No
Strength = 150MG
----
Product id = 18829
Application Number = 18343
Date of Application = 17,, Jan, 1985
RX/OTC/DISCN = RX
Tradename = CAPOTEN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PAR PHARM
ProductNo = 005
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 18831
Application Number = 18343
Date of Application = 17,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = CAPOTEN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PAR PHARM
ProductNo = 006
Tecode =
Rld = No
Strength = 37.5MG
----
Product id = 18833
Application Number = 18343
Date of Application = 13,, Jun, 1995
RX/OTC/DISCN = DISCN
Tradename = CAPOTEN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PAR PHARM
ProductNo = 007
Tecode =
Rld = No
Strength = 75MG
----
Product id = 18836
Application Number = 18709
Date of Application = 12,, Oct, 1984
RX/OTC/DISCN = DISCN
Tradename = CAPOZIDE 25/15
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 18837
Application Number = 18709
Date of Application = 12,, Oct, 1984
RX/OTC/DISCN = DISCN
Tradename = CAPOZIDE 25/25
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;25MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 18839
Application Number = 18709
Date of Application = 12,, Oct, 1984
RX/OTC/DISCN = DISCN
Tradename = CAPOZIDE 50/25
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTHECON
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG;25MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 18838
Application Number = 18709
Date of Application = 12,, Oct, 1984
RX/OTC/DISCN = DISCN
Tradename = CAPOZIDE 50/15
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTHECON
ProductNo = 004
Tecode =
Rld = No
Strength = 50MG;15MG **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 18898
Application Number = 74322
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 18901
Application Number = 74322
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18904
Application Number = 74322
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18907
Application Number = 74322
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 004
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18882
Application Number = 74363
Date of Application = 9,, Nov, 1995
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 18885
Application Number = 74363
Date of Application = 9,, Nov, 1995
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18888
Application Number = 74363
Date of Application = 9,, Nov, 1995
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18891
Application Number = 74363
Date of Application = 9,, Nov, 1995
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18918
Application Number = 74386
Date of Application = 23,, May, 1996
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 18921
Application Number = 74386
Date of Application = 23,, May, 1996
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18924
Application Number = 74386
Date of Application = 23,, May, 1996
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18927
Application Number = 74386
Date of Application = 23,, May, 1996
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18914
Application Number = 74418
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG
----
Product id = 18915
Application Number = 74418
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS LLC
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18916
Application Number = 74418
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS LLC
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18917
Application Number = 74418
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS LLC
ProductNo = 004
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18850
Application Number = 74423
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG
----
Product id = 18851
Application Number = 74423
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18852
Application Number = 74423
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18853
Application Number = 74423
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 004
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18899
Application Number = 74433
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG
----
Product id = 18902
Application Number = 74433
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18905
Application Number = 74433
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18908
Application Number = 74433
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 004
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18866
Application Number = 74434
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 18867
Application Number = 74434
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18868
Application Number = 74434
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18869
Application Number = 74434
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18919
Application Number = 74451
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG
----
Product id = 18922
Application Number = 74451
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18925
Application Number = 74451
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18928
Application Number = 74451
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 004
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18910
Application Number = 74462
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG
----
Product id = 18911
Application Number = 74462
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18912
Application Number = 74462
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18913
Application Number = 74462
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18846
Application Number = 74472
Date of Application = 31,, Mar, 1995
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG
----
Product id = 18847
Application Number = 74472
Date of Application = 31,, Mar, 1995
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18848
Application Number = 74472
Date of Application = 31,, Mar, 1995
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTHECON
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18849
Application Number = 74472
Date of Application = 31,, Mar, 1995
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTHECON
ProductNo = 004
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18874
Application Number = 74477
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG
----
Product id = 18875
Application Number = 74477
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18876
Application Number = 74477
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18877
Application Number = 74477
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 004
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18883
Application Number = 74481
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG
----
Product id = 18886
Application Number = 74481
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18889
Application Number = 74481
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18892
Application Number = 74481
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18900
Application Number = 74483
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG
----
Product id = 18903
Application Number = 74483
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18906
Application Number = 74483
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18909
Application Number = 74483
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 004
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18870
Application Number = 74493
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG
----
Product id = 18871
Application Number = 74493
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18872
Application Number = 74493
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18873
Application Number = 74493
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 004
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18858
Application Number = 74505
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 18859
Application Number = 74505
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS LLC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18860
Application Number = 74505
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS LLC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18861
Application Number = 74505
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA PHARMS LLC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18884
Application Number = 74519
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG
----
Product id = 18887
Application Number = 74519
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18890
Application Number = 74519
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18893
Application Number = 74519
Date of Application = 13,, Feb, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18930
Application Number = 74532
Date of Application = 28,, Mar, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 18931
Application Number = 74532
Date of Application = 28,, Mar, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18932
Application Number = 74532
Date of Application = 28,, Mar, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18933
Application Number = 74532
Date of Application = 28,, Mar, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 004
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18920
Application Number = 74576
Date of Application = 23,, Apr, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG
----
Product id = 18923
Application Number = 74576
Date of Application = 23,, Apr, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18926
Application Number = 74576
Date of Application = 23,, Apr, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18929
Application Number = 74576
Date of Application = 23,, Apr, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 004
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18864
Application Number = 74590
Date of Application = 30,, Aug, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18863
Application Number = 74590
Date of Application = 30,, Aug, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18865
Application Number = 74590
Date of Application = 30,, Aug, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18862
Application Number = 74590
Date of Application = 30,, Aug, 1996
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = 12.5MG
----
Product id = 18878
Application Number = 74640
Date of Application = 31,, Mar, 1997
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 12.5MG
----
Product id = 18879
Application Number = 74640
Date of Application = 31,, Mar, 1997
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18880
Application Number = 74640
Date of Application = 31,, Mar, 1997
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18881
Application Number = 74640
Date of Application = 31,, Mar, 1997
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PUREPAC PHARM
ProductNo = 004
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18896
Application Number = 74677
Date of Application = 30,, May, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = STASON
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18895
Application Number = 74677
Date of Application = 30,, May, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = STASON
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18897
Application Number = 74677
Date of Application = 30,, May, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = STASON
ProductNo = 003
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18894
Application Number = 74677
Date of Application = 30,, May, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = STASON
ProductNo = 004
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 18842
Application Number = 74737
Date of Application = 28,, Oct, 1998
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG
----
Product id = 18843
Application Number = 74737
Date of Application = 28,, Oct, 1998
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG
----
Product id = 18844
Application Number = 74737
Date of Application = 28,, Oct, 1998
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 18845
Application Number = 74737
Date of Application = 28,, Oct, 1998
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 004
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18856
Application Number = 74748
Date of Application = 29,, May, 1997
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EGIS PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 18855
Application Number = 74748
Date of Application = 29,, May, 1997
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EGIS PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG
----
Product id = 18857
Application Number = 74748
Date of Application = 29,, May, 1997
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EGIS PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18854
Application Number = 74748
Date of Application = 29,, May, 1997
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EGIS PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = 12.5MG
----
Product id = 18946
Application Number = 74788
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG
----
Product id = 18947
Application Number = 74788
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS LLC
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 18949
Application Number = 74788
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS LLC
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG;25MG
----
Product id = 18948
Application Number = 74788
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS LLC
ProductNo = 004
Tecode =
Rld = No
Strength = 50MG;15MG
----
Product id = 18942
Application Number = 74827
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;15MG
----
Product id = 18943
Application Number = 74827
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 25MG;25MG
----
Product id = 18945
Application Number = 74827
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG;25MG
----
Product id = 18944
Application Number = 74827
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 004
Tecode = AB
Rld = No
Strength = 50MG;15MG
----
Product id = 18950
Application Number = 74832
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;25MG
----
Product id = 18938
Application Number = 74896
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 25MG;15MG
----
Product id = 18939
Application Number = 74896
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 25MG;25MG
----
Product id = 18941
Application Number = 74896
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 50MG;25MG
----
Product id = 18940
Application Number = 74896
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = RX
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = Yes
Strength = 50MG;15MG
----
Product id = 18934
Application Number = 75055
Date of Application = 18,, Jun, 1998
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;15MG
----
Product id = 18935
Application Number = 75055
Date of Application = 18,, Jun, 1998
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;25MG
----
Product id = 18937
Application Number = 75055
Date of Application = 18,, Jun, 1998
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG;25MG
----
Product id = 18936
Application Number = 75055
Date of Application = 18,, Jun, 1998
RX/OTC/DISCN = DISCN
Tradename = CAPTOPRIL AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = 50MG;15MG
----