carbamazepine
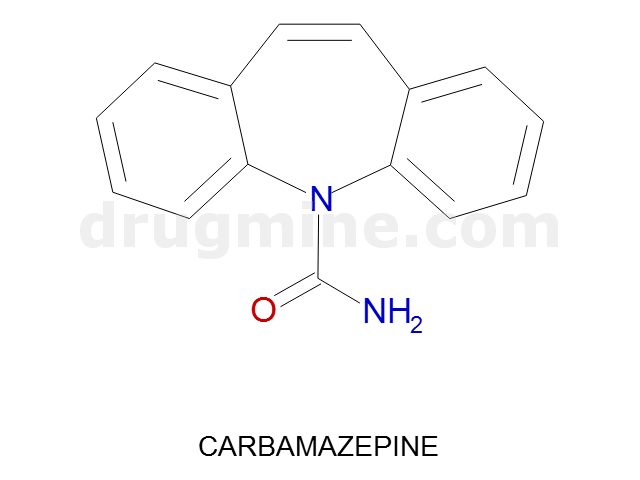

Name: CARBAMAZEPINE
ID :
MW: 236
Number of atoms: 18
Molecular_Formula: C15H12N2O
Alogp: 2.679
Indication class : Analgesic; Anticonvulsant
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
carbamazepine containing products summary
There are in total 48 different products containing the active ingredient carbamazepine. From the 48 drug products, 8 have been discontinued.Product id = 28490
Application Number = 16608
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TEGRETOL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 200MG
----
Product id = 15368
Application Number = 18281
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = TEGRETOL
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 100MG
----
Product id = 14816
Application Number = 18927
Date of Application = 18,, Dec, 1987
RX/OTC/DISCN = RX
Tradename = TEGRETOL
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 100MG/5ML
----
Product id = 16663
Application Number = 20234
Date of Application = 25,, Mar, 1996
RX/OTC/DISCN = RX
Tradename = TEGRETOL-XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 16664
Application Number = 20234
Date of Application = 25,, Mar, 1996
RX/OTC/DISCN = RX
Tradename = TEGRETOL-XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 16665
Application Number = 20234
Date of Application = 25,, Mar, 1996
RX/OTC/DISCN = RX
Tradename = TEGRETOL-XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 400MG
----
Product id = 349
Application Number = 20712
Date of Application = 30,, Sep, 1997
RX/OTC/DISCN = RX
Tradename = CARBATROL
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = SHIRE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 350
Application Number = 20712
Date of Application = 30,, Sep, 1997
RX/OTC/DISCN = RX
Tradename = CARBATROL
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = SHIRE
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 300MG
----
Product id = 348
Application Number = 20712
Date of Application = 30,, Sep, 1997
RX/OTC/DISCN = RX
Tradename = CARBATROL
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = SHIRE
ProductNo = 003
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 509
Application Number = 21710
Date of Application = 10,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = EQUETRO
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = VALIDUS PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 510
Application Number = 21710
Date of Application = 10,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = EQUETRO
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = VALIDUS PHARMS INC
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG
----
Product id = 511
Application Number = 21710
Date of Application = 10,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = EQUETRO
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = N
Applicant Name = VALIDUS PHARMS INC
ProductNo = 003
Tecode =
Rld = Yes
Strength = 300MG
----
Product id = 18955
Application Number = 70231
Date of Application = 14,, Aug, 1986
RX/OTC/DISCN = DISCN
Tradename = CARBAMAZEPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INWOOD LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 18962
Application Number = 70300
Date of Application = 15,, May, 1986
RX/OTC/DISCN = DISCN
Tradename = CARBAMAZEPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 18963
Application Number = 70429
Date of Application = 2,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = CARBAMAZEPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 20892
Application Number = 70541
Date of Application = 17,, Sep, 1986
RX/OTC/DISCN = RX
Tradename = EPITOL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 18956
Application Number = 71479
Date of Application = 24,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = CARBAMAZEPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 18953
Application Number = 71696
Date of Application = 9,, Nov, 1987
RX/OTC/DISCN = DISCN
Tradename = CARBAMAZEPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 15222
Application Number = 71940
Date of Application = 1,, Feb, 1988
RX/OTC/DISCN = DISCN
Tradename = CARBAMAZEPINE
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = JUBILANT CADISTA
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 15250
Application Number = 73524
Date of Application = 29,, Jul, 1992
RX/OTC/DISCN = RX
Tradename = EPITOL
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 18957
Application Number = 74649
Date of Application = 3,, Oct, 1996
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 15223
Application Number = 75687
Date of Application = 24,, Oct, 2000
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = TARO PHARM INDS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 15224
Application Number = 75687
Date of Application = 29,, Jul, 2002
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = TARO PHARM INDS
ProductNo = 002
Tecode =
Rld = Yes
Strength = 200MG
----
Product id = 15225
Application Number = 75712
Date of Application = 5,, Jul, 2001
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 14651
Application Number = 75714
Date of Application = 5,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG/5ML
----
Product id = 14650
Application Number = 75875
Date of Application = 21,, Dec, 2000
RX/OTC/DISCN = DISCN
Tradename = CARBAMAZEPINE
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/5ML
----
Product id = 18954
Application Number = 75948
Date of Application = 27,, Feb, 2002
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 28597
Application Number = 76525
Date of Application = 26,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = TERIL
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 200MG
----
Product id = 339
Application Number = 76697
Date of Application = 20,, May, 2011
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = NOSTRUM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 340
Application Number = 76697
Date of Application = 20,, May, 2011
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = NOSTRUM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 341
Application Number = 76697
Date of Application = 20,, May, 2011
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = NOSTRUM
ProductNo = 003
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 14818
Application Number = 76729
Date of Application = 20,, Sep, 2004
RX/OTC/DISCN = RX
Tradename = TERIL
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG/5ML
----
Product id = 18958
Application Number = 77272
Date of Application = 7,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG
----
Product id = 18959
Application Number = 77272
Date of Application = 7,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 18960
Application Number = 77272
Date of Application = 7,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 300MG
----
Product id = 18961
Application Number = 77272
Date of Application = 7,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = 400MG
----
Product id = 15778
Application Number = 78115
Date of Application = 31,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 15779
Application Number = 78115
Date of Application = 31,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = TARO
ProductNo = 002
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 15780
Application Number = 78115
Date of Application = 31,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = TARO
ProductNo = 003
Tecode = AB
Rld = No
Strength = 400MG
----
Product id = 345
Application Number = 78592
Date of Application = 20,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 346
Application Number = 78592
Date of Application = 20,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 347
Application Number = 78592
Date of Application = 20,, Sep, 2012
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 336
Application Number = 78986
Date of Application = 25,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 337
Application Number = 78986
Date of Application = 25,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 338
Application Number = 78986
Date of Application = 25,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 342
Application Number = 201106
Date of Application = 21,, Jun, 2013
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 343
Application Number = 201106
Date of Application = 21,, Jun, 2013
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TARO
ProductNo = 002
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 344
Application Number = 201106
Date of Application = 21,, Jun, 2013
RX/OTC/DISCN = RX
Tradename = CARBAMAZEPINE
Route/format = ORAL / CAPSULE, EXTENDED RELEASE
Application Type = A
Applicant Name = TARO
ProductNo = 003
Tecode = AB
Rld = No
Strength = 300MG
----