cefotaxime-sodium
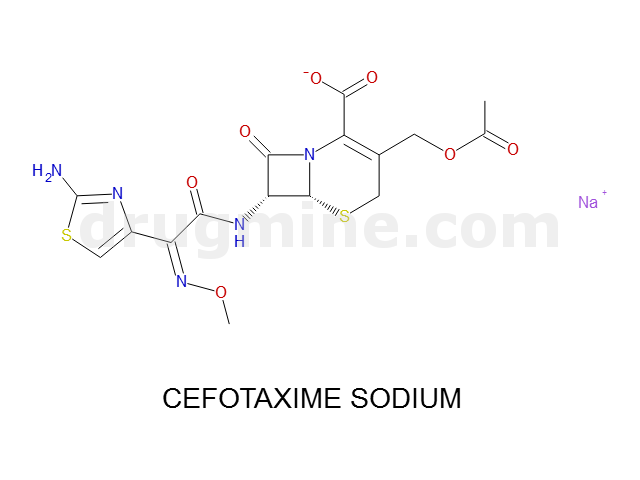
Name: CEFOTAXIME SODIUM
ID :
MW: 454
Number of atoms: 30
Molecular_Formula: C16H16N5O7S2
Alogp: -2.384
Indication class : Antibacterial
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
cefotaxime sodium containing products summary
There are in total 38 different products containing the active ingredient cefotaxime sodium. From the 38 drug products, 14 have been discontinued.Product id = 6741
Application Number = 50547
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CLAFORAN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 500MG BASE/VIAL
----
Product id = 6737
Application Number = 50547
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CLAFORAN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 1GM BASE/VIAL
----
Product id = 6739
Application Number = 50547
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CLAFORAN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = EQ 2GM BASE/VIAL
----
Product id = 6740
Application Number = 50547
Date of Application = 29,, Dec, 1983
RX/OTC/DISCN = RX
Tradename = CLAFORAN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 004
Tecode = AP
Rld = Yes
Strength = EQ 10GM BASE/VIAL
----
Product id = 6744
Application Number = 50596
Date of Application = 20,, May, 1985
RX/OTC/DISCN = DISCN
Tradename = CLAFORAN IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 20MG BASE/ML
----
Product id = 6742
Application Number = 50596
Date of Application = 20,, May, 1985
RX/OTC/DISCN = RX
Tradename = CLAFORAN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 20MG BASE/ML
----
Product id = 6745
Application Number = 50596
Date of Application = 20,, May, 1985
RX/OTC/DISCN = DISCN
Tradename = CLAFORAN IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 40MG BASE/ML
----
Product id = 6743
Application Number = 50596
Date of Application = 20,, May, 1985
RX/OTC/DISCN = RX
Tradename = CLAFORAN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 004
Tecode =
Rld = Yes
Strength = EQ 40MG BASE/ML
----
Product id = 6447
Application Number = 50792
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = CEFOTAXIME AND DEXTROSE 2.4% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2GM BASE
----
Product id = 6448
Application Number = 50792
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = DISCN
Tradename = CEFOTAXIME AND DEXTROSE 3.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1GM BASE
----
Product id = 6736
Application Number = 62659
Date of Application = 13,, Jan, 1987
RX/OTC/DISCN = RX
Tradename = CLAFORAN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 1GM BASE/VIAL
----
Product id = 6738
Application Number = 62659
Date of Application = 13,, Jan, 1987
RX/OTC/DISCN = RX
Tradename = CLAFORAN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 2GM BASE/VIAL
----
Product id = 6441
Application Number = 64200
Date of Application = 24,, Mar, 2000
RX/OTC/DISCN = DISCN
Tradename = CEFOTAXIME
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6437
Application Number = 64200
Date of Application = 24,, Mar, 2000
RX/OTC/DISCN = DISCN
Tradename = CEFOTAXIME
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6438
Application Number = 64200
Date of Application = 24,, Mar, 2000
RX/OTC/DISCN = DISCN
Tradename = CEFOTAXIME
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6439
Application Number = 64201
Date of Application = 24,, Mar, 2000
RX/OTC/DISCN = DISCN
Tradename = CEFOTAXIME
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6440
Application Number = 64201
Date of Application = 24,, Mar, 2000
RX/OTC/DISCN = DISCN
Tradename = CEFOTAXIME
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20GM BASE/VIAL
----
Product id = 6444
Application Number = 65071
Date of Application = 20,, Nov, 2002
RX/OTC/DISCN = RX
Tradename = CEFOTAXIME
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6445
Application Number = 65072
Date of Application = 20,, Nov, 2002
RX/OTC/DISCN = RX
Tradename = CEFOTAXIME
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6442
Application Number = 65072
Date of Application = 20,, Nov, 2002
RX/OTC/DISCN = RX
Tradename = CEFOTAXIME
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6443
Application Number = 65072
Date of Application = 20,, Nov, 2002
RX/OTC/DISCN = RX
Tradename = CEFOTAXIME
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6462
Application Number = 65124
Date of Application = 24,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = CEFOTAXIME SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUPIN
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6460
Application Number = 65124
Date of Application = 24,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = CEFOTAXIME SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUPIN
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6461
Application Number = 65124
Date of Application = 24,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = CEFOTAXIME SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUPIN
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6446
Application Number = 65197
Date of Application = 29,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = CEFOTAXIME
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6464
Application Number = 65197
Date of Application = 20,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = CEFOTAXIME SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6463
Application Number = 65197
Date of Application = 20,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = CEFOTAXIME SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6459
Application Number = 65290
Date of Application = 11,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = CEFOTAXIME SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6454
Application Number = 65290
Date of Application = 11,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = CEFOTAXIME SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6456
Application Number = 65290
Date of Application = 11,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = CEFOTAXIME SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6458
Application Number = 65292
Date of Application = 10,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = CEFOTAXIME SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6455
Application Number = 65293
Date of Application = 10,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = CEFOTAXIME SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6457
Application Number = 65293
Date of Application = 10,, Aug, 2006
RX/OTC/DISCN = RX
Tradename = CEFOTAXIME SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6453
Application Number = 65348
Date of Application = 25,, Jan, 2010
RX/OTC/DISCN = DISCN
Tradename = CEFOTAXIME SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CEPHAZONE PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6452
Application Number = 65516
Date of Application = 6,, Nov, 2009
RX/OTC/DISCN = DISCN
Tradename = CEFOTAXIME SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6451
Application Number = 65517
Date of Application = 6,, Nov, 2009
RX/OTC/DISCN = DISCN
Tradename = CEFOTAXIME SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6449
Application Number = 65517
Date of Application = 6,, Nov, 2009
RX/OTC/DISCN = DISCN
Tradename = CEFOTAXIME SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6450
Application Number = 65517
Date of Application = 6,, Nov, 2009
RX/OTC/DISCN = DISCN
Tradename = CEFOTAXIME SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----