cefoxitin-sodium
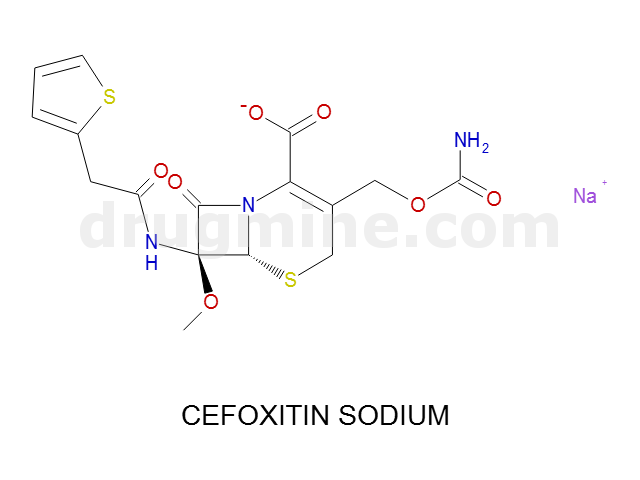
Name: CEFOXITIN SODIUM
ID :
MW: 426
Number of atoms: 28
Molecular_Formula: C16H16N3O7S2
Alogp: -1.151
Indication class : Antibacterial
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
cefoxitin sodium containing products summary
There are in total 31 different products containing the active ingredient cefoxitin sodium. From the 31 drug products, 12 have been discontinued.Product id = 9064
Application Number = 50517
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MEFOXIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = MYLAN INSTITUTIONAL
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 9066
Application Number = 50517
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MEFOXIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = MYLAN INSTITUTIONAL
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 9067
Application Number = 50517
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = MEFOXIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = MYLAN INSTITUTIONAL
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 9073
Application Number = 50581
Date of Application = 20,, Sep, 1984
RX/OTC/DISCN = DISCN
Tradename = MEFOXIN IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 40MG BASE/ML
----
Product id = 9072
Application Number = 50581
Date of Application = 20,, Sep, 1984
RX/OTC/DISCN = DISCN
Tradename = MEFOXIN IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = MERCK
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20MG BASE/ML
----
Product id = 9068
Application Number = 50581
Date of Application = 20,, Sep, 1984
RX/OTC/DISCN = DISCN
Tradename = MEFOXIN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = MERCK
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 20MG BASE/ML
----
Product id = 9069
Application Number = 50581
Date of Application = 20,, Sep, 1984
RX/OTC/DISCN = DISCN
Tradename = MEFOXIN IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = MERCK
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 40MG BASE/ML
----
Product id = 9063
Application Number = 62757
Date of Application = 8,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = MEFOXIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MYLAN INSTITUTIONAL
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 9065
Application Number = 62757
Date of Application = 8,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = MEFOXIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MYLAN INSTITUTIONAL
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 9070
Application Number = 63182
Date of Application = 25,, Jan, 1993
RX/OTC/DISCN = RX
Tradename = MEFOXIN IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MYLAN INSTITUTIONAL
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 20MG BASE/ML
----
Product id = 9071
Application Number = 63182
Date of Application = 25,, Jan, 1993
RX/OTC/DISCN = RX
Tradename = MEFOXIN IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MYLAN INSTITUTIONAL
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 40MG BASE/ML
----
Product id = 6481
Application Number = 65011
Date of Application = 3,, Jul, 2000
RX/OTC/DISCN = DISCN
Tradename = CEFOXITIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6479
Application Number = 65012
Date of Application = 3,, Jul, 2000
RX/OTC/DISCN = DISCN
Tradename = CEFOXITIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6480
Application Number = 65012
Date of Application = 3,, Jul, 2000
RX/OTC/DISCN = DISCN
Tradename = CEFOXITIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6487
Application Number = 65050
Date of Application = 11,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = CEFOXITIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6485
Application Number = 65051
Date of Application = 11,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = CEFOXITIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6486
Application Number = 65051
Date of Application = 11,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = CEFOXITIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6491
Application Number = 65214
Date of Application = 10,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = CEFOXITIN AND DEXTROSE IN DUPLEX CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = B BRAUN
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6492
Application Number = 65214
Date of Application = 10,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = CEFOXITIN AND DEXTROSE IN DUPLEX CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = B BRAUN
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6482
Application Number = 65238
Date of Application = 12,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = CEFOXITIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6483
Application Number = 65238
Date of Application = 12,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = CEFOXITIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6484
Application Number = 65239
Date of Application = 2,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = CEFOXITIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6490
Application Number = 65312
Date of Application = 13,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = CEFOXITIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6488
Application Number = 65313
Date of Application = 23,, Jan, 2006
RX/OTC/DISCN = RX
Tradename = CEFOXITIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6489
Application Number = 65313
Date of Application = 23,, Jan, 2006
RX/OTC/DISCN = RX
Tradename = CEFOXITIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6473
Application Number = 65414
Date of Application = 12,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = CEFOXITIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 1GM BASE/VIAL
----
Product id = 6474
Application Number = 65414
Date of Application = 12,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = CEFOXITIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 2GM BASE/VIAL
----
Product id = 6475
Application Number = 65415
Date of Application = 19,, May, 2010
RX/OTC/DISCN = RX
Tradename = CEFOXITIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 10GM BASE/VIAL
----
Product id = 6478
Application Number = 65464
Date of Application = 31,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = CEFOXITIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ANTIBIOTICOS BRASIL
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6476
Application Number = 65467
Date of Application = 31,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = CEFOXITIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ANTIBIOTICOS BRASIL
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6477
Application Number = 65467
Date of Application = 31,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = CEFOXITIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ANTIBIOTICOS BRASIL
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----