cytarabine
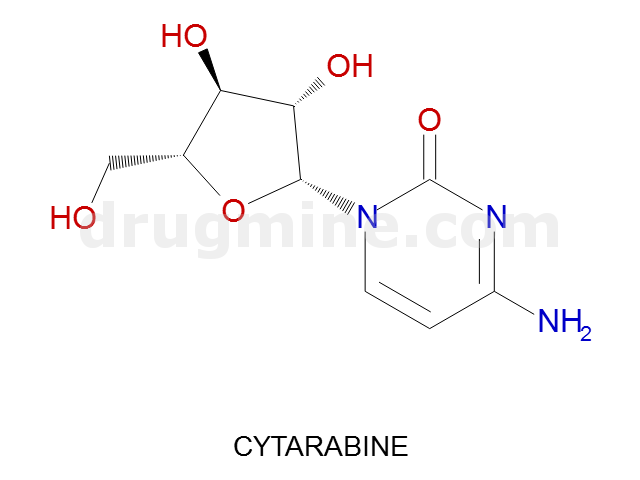
Name: CYTARABINE
ID :
MW: 243
Number of atoms: 17
Molecular_Formula: C9H13N3O5
Alogp: -2.396
Indication class : Antiviral; Antineoplastic
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
cytarabine containing products summary
There are in total 22 different products containing the active ingredient cytarabine. From the 22 drug products, 4 have been discontinued.Product id = 6925
Application Number = 16793
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CYTARABINE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = TEVA PARENTERAL
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/VIAL
----
Product id = 6926
Application Number = 16793
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CYTARABINE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = TEVA PARENTERAL
ProductNo = 002
Tecode =
Rld = No
Strength = 500MG/VIAL
----
Product id = 6923
Application Number = 16793
Date of Application = 21,, Dec, 1987
RX/OTC/DISCN = DISCN
Tradename = CYTARABINE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = TEVA PARENTERAL
ProductNo = 003
Tecode =
Rld = No
Strength = 1GM/VIAL
----
Product id = 6924
Application Number = 16793
Date of Application = 21,, Dec, 1987
RX/OTC/DISCN = DISCN
Tradename = CYTARABINE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = TEVA PARENTERAL
ProductNo = 004
Tecode =
Rld = No
Strength = 2GM/VIAL
----
Product id = 5559
Application Number = 21041
Date of Application = 1,, Apr, 1999
RX/OTC/DISCN = RX
Tradename = DEPOCYT
Route/format = INJECTION / INJECTABLE, LIPOSOMAL
Application Type = N
Applicant Name = PACIRA PHARMS INC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 10MG/ML
----
Product id = 6912
Application Number = 71471
Date of Application = 2,, Aug, 1989
RX/OTC/DISCN = RX
Tradename = CYTARABINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EUROHLTH INTL SARL
ProductNo = 001
Tecode = AP
Rld = No
Strength = 100MG/VIAL
----
Product id = 6913
Application Number = 71472
Date of Application = 2,, Aug, 1989
RX/OTC/DISCN = RX
Tradename = CYTARABINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EUROHLTH INTL SARL
ProductNo = 001
Tecode = AP
Rld = No
Strength = 500MG/VIAL
----
Product id = 6915
Application Number = 71868
Date of Application = 4,, Jun, 1990
RX/OTC/DISCN = RX
Tradename = CYTARABINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 20MG/ML
----
Product id = 6916
Application Number = 72168
Date of Application = 31,, Aug, 1990
RX/OTC/DISCN = RX
Tradename = CYTARABINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 20MG/ML
----
Product id = 6917
Application Number = 72945
Date of Application = 28,, Feb, 1994
RX/OTC/DISCN = RX
Tradename = CYTARABINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 20MG/ML
----
Product id = 6910
Application Number = 74245
Date of Application = 31,, Aug, 1994
RX/OTC/DISCN = RX
Tradename = CYTARABINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EUROHLTH INTL SARL
ProductNo = 001
Tecode = AP
Rld = No
Strength = 1GM/VIAL
----
Product id = 6911
Application Number = 74245
Date of Application = 31,, Aug, 1994
RX/OTC/DISCN = RX
Tradename = CYTARABINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EUROHLTH INTL SARL
ProductNo = 002
Tecode = AP
Rld = No
Strength = 2GM/VIAL
----
Product id = 6929
Application Number = 75206
Date of Application = 30,, Dec, 1998
RX/OTC/DISCN = RX
Tradename = CYTOSAR-U
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 100MG/VIAL
----
Product id = 6930
Application Number = 75206
Date of Application = 30,, Dec, 1998
RX/OTC/DISCN = RX
Tradename = CYTOSAR-U
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = 500MG/VIAL
----
Product id = 6928
Application Number = 75206
Date of Application = 30,, Dec, 1998
RX/OTC/DISCN = RX
Tradename = CYTOSAR-U
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = 2GM/VIAL
----
Product id = 6927
Application Number = 75206
Date of Application = 30,, Dec, 1998
RX/OTC/DISCN = RX
Tradename = CYTOSAR-U
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 004
Tecode = AP
Rld = Yes
Strength = 1GM/VIAL
----
Product id = 6918
Application Number = 75383
Date of Application = 22,, Nov, 1999
RX/OTC/DISCN = RX
Tradename = CYTARABINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 100MG/ML
----
Product id = 6914
Application Number = 76512
Date of Application = 15,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = CYTARABINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 100MG/ML
----
Product id = 6919
Application Number = 200914
Date of Application = 13,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = CYTARABINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ONCO THERAPIES LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 20MG/ML
----
Product id = 6920
Application Number = 200915
Date of Application = 13,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = CYTARABINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ONCO THERAPIES LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 20MG/ML
----
Product id = 6921
Application Number = 200916
Date of Application = 13,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = CYTARABINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ONCO THERAPIES LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 20MG/ML
----
Product id = 6922
Application Number = 201784
Date of Application = 30,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = CYTARABINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ONCO THERAPIES LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 100MG/ML
----