daunorubicin-hydrochloride
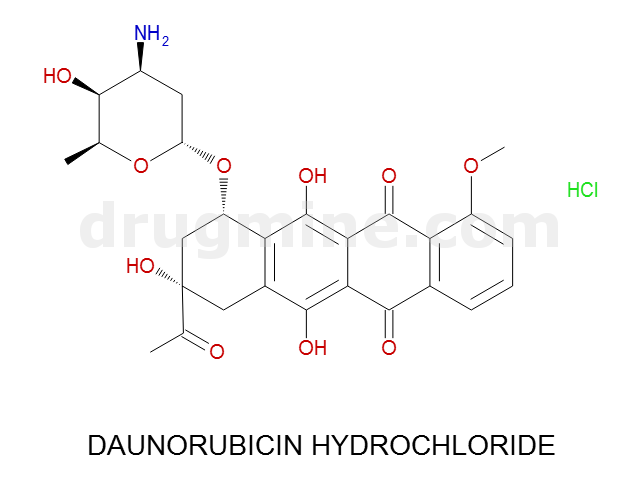
Name: DAUNORUBICIN HYDROCHLORIDE
ID :
MW: 528
Number of atoms: 38
Molecular_Formula: C27H29NO10
Alogp: 0.628
Indication class : Antineoplastic
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
daunorubicin hydrochloride containing products summary
There are in total 9 different products containing the active ingredient daunorubicin hydrochloride. From the 9 drug products, 4 have been discontinued.Product id = 6597
Application Number = 50484
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CERUBIDINE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = WYETH AYERST
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 20MG BASE/VIAL
----
Product id = 6956
Application Number = 50731
Date of Application = 30,, Jan, 1998
RX/OTC/DISCN = RX
Tradename = DAUNORUBICIN HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = EUROHLTH INTL SARL
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 5MG BASE/ML
----
Product id = 6596
Application Number = 61876
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CERUBIDINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 20MG BASE/VIAL
----
Product id = 6595
Application Number = 64103
Date of Application = 3,, Feb, 1995
RX/OTC/DISCN = RX
Tradename = CERUBIDINE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EUROHLTH INTL SARL
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 20MG BASE/VIAL
----
Product id = 6959
Application Number = 64212
Date of Application = 23,, Jun, 1998
RX/OTC/DISCN = DISCN
Tradename = DAUNORUBICIN HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PARENTERAL
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 20MG BASE/VIAL
----
Product id = 6960
Application Number = 64212
Date of Application = 3,, May, 1999
RX/OTC/DISCN = DISCN
Tradename = DAUNORUBICIN HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PARENTERAL
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 50MG BASE/VIAL
----
Product id = 6958
Application Number = 65000
Date of Application = 25,, May, 1999
RX/OTC/DISCN = RX
Tradename = DAUNORUBICIN HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 20MG BASE/VIAL
----
Product id = 6957
Application Number = 65034
Date of Application = 20,, Nov, 2001
RX/OTC/DISCN = RX
Tradename = DAUNORUBICIN HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE/VIAL
----
Product id = 6961
Application Number = 65035
Date of Application = 24,, Jan, 2000
RX/OTC/DISCN = RX
Tradename = DAUNORUBICIN HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 5MG BASE/ML
----