fluocinolone-acetonide
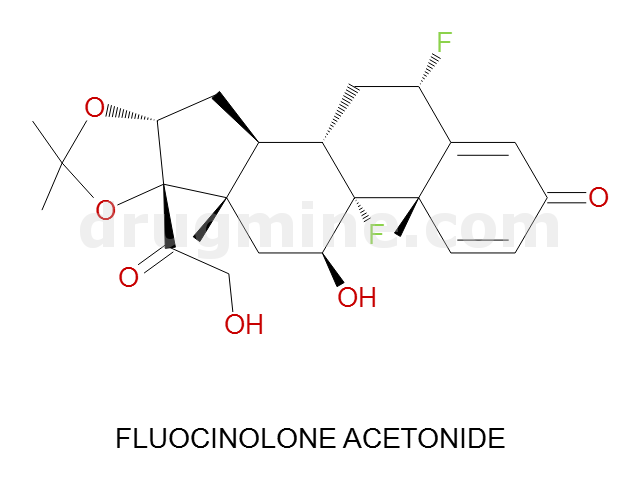
Name: FLUOCINOLONE ACETONIDE
ID :
MW: 452
Number of atoms: 32
Molecular_Formula: C24H30F2O6
Alogp: 0.995
Indication class : Glucocorticoid
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
fluocinolone acetonide containing products summary
There are in total 58 different products containing the active ingredient fluocinolone acetonide. From the 58 drug products, 30 have been discontinued.Product id = 4386
Application Number = 12787
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = SYNALAR
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = MEDIMETRIKS PHARMS
ProductNo = 002
Tecode = AT
Rld = Yes
Strength = 0.025%
----
Product id = 4385
Application Number = 12787
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = SYNALAR
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = MEDIMETRIKS PHARMS
ProductNo = 004
Tecode = AT
Rld = Yes
Strength = 0.01%
----
Product id = 4387
Application Number = 12787
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = SYNALAR
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = MEDIMETRIKS PHARMS
ProductNo = 005
Tecode = AT
Rld = Yes
Strength = 0.025%
----
Product id = 12575
Application Number = 13960
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = SYNALAR
Route/format = TOPICAL / OINTMENT
Application Type = N
Applicant Name = MEDIMETRIKS PHARMS
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.025%
----
Product id = 14280
Application Number = 15296
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = SYNALAR
Route/format = TOPICAL / SOLUTION
Application Type = N
Applicant Name = MEDIMETRIKS PHARMS
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.01%
----
Product id = 4388
Application Number = 16161
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = SYNALAR-HP
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = MEDIMETRIKS PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.2%
----
Product id = 12284
Application Number = 19452
Date of Application = 3,, Feb, 1988
RX/OTC/DISCN = RX
Tradename = DERMA-SMOOTHE/FS
Route/format = TOPICAL / OIL
Application Type = N
Applicant Name = HILL DERMAC
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.01%
----
Product id = 12285
Application Number = 19452
Date of Application = 9,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = DERMA-SMOOTHE/FS
Route/format = TOPICAL / OIL
Application Type = N
Applicant Name = HILL DERMAC
ProductNo = 002
Tecode = AT
Rld = Yes
Strength = 0.01%
----
Product id = 12281
Application Number = 19452
Date of Application = 9,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = DERMOTIC
Route/format = OTIC / OIL/DROPS
Application Type = N
Applicant Name = HILL DERMAC
ProductNo = 003
Tecode = AT
Rld = Yes
Strength = 0.01%
----
Product id = 12809
Application Number = 20001
Date of Application = 27,, Aug, 1990
RX/OTC/DISCN = RX
Tradename = CAPEX
Route/format = TOPICAL / SHAMPOO
Application Type = N
Applicant Name = GALDERMA LABS LP
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.01%
----
Product id = 4405
Application Number = 21112
Date of Application = 18,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = TRI-LUMA
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = GALDERMA LABS LP
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.01%;4%;0.05%
----
Product id = 5547
Application Number = 21737
Date of Application = 8,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = RETISERT
Route/format = INTRAVITREAL / IMPLANT
Application Type = N
Applicant Name = BAUSCH AND LOMB
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.59MG
----
Product id = 4159
Application Number = 40035
Date of Application = 31,, Oct, 1994
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 0.01%
----
Product id = 12460
Application Number = 40041
Date of Application = 15,, Sep, 1994
RX/OTC/DISCN = RX
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.025%
----
Product id = 4161
Application Number = 40042
Date of Application = 31,, Oct, 1994
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 14195
Application Number = 40059
Date of Application = 20,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = BAUSCH AND LOMB
ProductNo = 001
Tecode =
Rld = No
Strength = 0.01%
----
Product id = 4335
Application Number = 60700
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = NEO-SYNALAR
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = MEDIMETRIKS PHARMS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.025%;EQ 3.5MG BASE/GM
----
Product id = 4153
Application Number = 86810
Date of Application = 4,, Mar, 1982
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode =
Rld = No
Strength = 0.01%
----
Product id = 4154
Application Number = 86811
Date of Application = 4,, Mar, 1982
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4160
Application Number = 87102
Date of Application = 27,, Apr, 1982
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = 0.01%
----
Product id = 4162
Application Number = 87104
Date of Application = 27,, Apr, 1982
RX/OTC/DISCN = RX
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.025%
----
Product id = 4179
Application Number = 87156
Date of Application = 6,, Sep, 1984
RX/OTC/DISCN = DISCN
Tradename = FLUONID
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ALLERGAN HERBERT
ProductNo = 002
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 12465
Application Number = 87157
Date of Application = 6,, Sep, 1984
RX/OTC/DISCN = DISCN
Tradename = FLUONID
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = ALLERGAN HERBERT
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 14207
Application Number = 87158
Date of Application = 17,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = FLUONID
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = ALLERGAN HERBERT
ProductNo = 001
Tecode =
Rld = No
Strength = 0.01%
----
Product id = 14194
Application Number = 87159
Date of Application = 16,, Jun, 1982
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = ALPHARMA US PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.01%
----
Product id = 5407
Application Number = 87300
Date of Application = 27,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = FLUONID
Route/format = TOPICAL / GEL
Application Type = A
Applicant Name = ALLERGAN HERBERT
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4156
Application Number = 88045
Date of Application = 16,, Dec, 1982
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PHARMADERM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 12458
Application Number = 88046
Date of Application = 16,, Dec, 1982
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = PHARMADERM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4155
Application Number = 88047
Date of Application = 16,, Dec, 1982
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PHARMADERM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.01%
----
Product id = 14198
Application Number = 88048
Date of Application = 16,, Dec, 1982
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = PHARMADERM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.01%
----
Product id = 14196
Application Number = 88167
Date of Application = 16,, Dec, 1982
RX/OTC/DISCN = RX
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = FOUGERA
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.01%
----
Product id = 12456
Application Number = 88168
Date of Application = 16,, Dec, 1982
RX/OTC/DISCN = RX
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = FOUGERA
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.025%
----
Product id = 4150
Application Number = 88169
Date of Application = 16,, Dec, 1982
RX/OTC/DISCN = RX
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = FOUGERA
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.025%
----
Product id = 4149
Application Number = 88170
Date of Application = 16,, Dec, 1982
RX/OTC/DISCN = RX
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = FOUGERA
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.01%
----
Product id = 14211
Application Number = 88171
Date of Application = 9,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = FLUOTREX
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = SAVAGE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.01%
----
Product id = 12466
Application Number = 88172
Date of Application = 9,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = FLUOTREX
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = SAVAGE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4184
Application Number = 88173
Date of Application = 9,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = FLUOTREX
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = SAVAGE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4183
Application Number = 88174
Date of Application = 6,, May, 1983
RX/OTC/DISCN = DISCN
Tradename = FLUOTREX
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = SAVAGE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.01%
----
Product id = 14197
Application Number = 88312
Date of Application = 27,, Jan, 1984
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = MORTON GROVE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.01%
----
Product id = 4147
Application Number = 88360
Date of Application = 16,, Jan, 1984
RX/OTC/DISCN = DISCN
Tradename = FLUOCET
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ALPHARMA US PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4148
Application Number = 88361
Date of Application = 16,, Jan, 1984
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ALPHARMA US PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.01%
----
Product id = 14199
Application Number = 88449
Date of Application = 8,, Feb, 1984
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = PHARMAFAIR
ProductNo = 001
Tecode =
Rld = No
Strength = 0.01%
----
Product id = 4157
Application Number = 88499
Date of Application = 2,, Aug, 1984
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PHARMAFAIR
ProductNo = 001
Tecode =
Rld = No
Strength = 0.01%
----
Product id = 4158
Application Number = 88506
Date of Application = 2,, Aug, 1984
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PHARMAFAIR
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 12459
Application Number = 88507
Date of Application = 27,, Feb, 1984
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = PHARMAFAIR
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 12461
Application Number = 88742
Date of Application = 8,, Feb, 1985
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4164
Application Number = 88756
Date of Application = 28,, Mar, 1985
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.025%
----
Product id = 4163
Application Number = 88757
Date of Application = 11,, Feb, 1985
RX/OTC/DISCN = DISCN
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.01%
----
Product id = 14200
Application Number = 89124
Date of Application = 11,, Sep, 1985
RX/OTC/DISCN = RX
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.01%
----
Product id = 12457
Application Number = 89524
Date of Application = 26,, Jul, 1988
RX/OTC/DISCN = RX
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = G AND W LABS
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.025%
----
Product id = 4152
Application Number = 89525
Date of Application = 26,, Jul, 1988
RX/OTC/DISCN = RX
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = G AND W LABS
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.025%
----
Product id = 4151
Application Number = 89526
Date of Application = 26,, Jul, 1988
RX/OTC/DISCN = RX
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = G AND W LABS
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.01%
----
Product id = 12282
Application Number = 91306
Date of Application = 17,, Oct, 2011
RX/OTC/DISCN = RX
Tradename = FLUOCINOLONE ACETONIDE
Route/format = OTIC / OIL/DROPS
Application Type = A
Applicant Name = IDENTI PHARMS INC
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.01%
----
Product id = 12286
Application Number = 201759
Date of Application = 17,, Oct, 2011
RX/OTC/DISCN = RX
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / OIL
Application Type = A
Applicant Name = IDENTI PHARMS INC
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.01%
----
Product id = 12287
Application Number = 201764
Date of Application = 17,, Oct, 2011
RX/OTC/DISCN = RX
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / OIL
Application Type = A
Applicant Name = IDENTI PHARMS INC
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.01%
----
Product id = 5545
Application Number = 201923
Date of Application = 26,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = ILUVIEN
Route/format = INTRAVITREAL / IMPLANT
Application Type = N
Applicant Name = ALIMERA SCIENCES INC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.19MG
----
Product id = 12288
Application Number = 202847
Date of Application = 9,, Aug, 2013
RX/OTC/DISCN = RX
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / OIL
Application Type = A
Applicant Name = PERRIGO ISRAEL
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.01%
----
Product id = 12289
Application Number = 202848
Date of Application = 9,, Aug, 2013
RX/OTC/DISCN = RX
Tradename = FLUOCINOLONE ACETONIDE
Route/format = TOPICAL / OIL
Application Type = A
Applicant Name = PERRIGO ISRAEL
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.01%
----