fosinopril-sodium

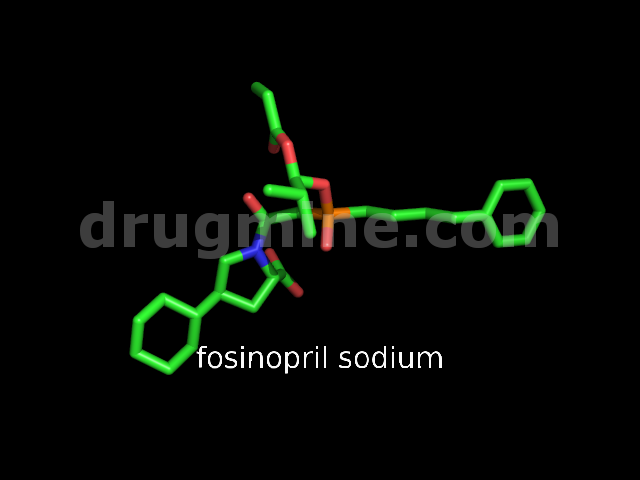
Name: FOSINOPRIL SODIUM
ID :
MW: 563
Number of atoms: 39
Molecular_Formula: C30H45NO7P
Alogp: 4.129
Indication class : Antihypertensive; Enzyme Inhibitor (angiotensin-converting)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
fosinopril sodium containing products summary
There are in total 53 different products containing the active ingredient fosinopril sodium. From the 53 drug products, 23 have been discontinued.Product id = 25068
Application Number = 19915
Date of Application = 16,, May, 1991
RX/OTC/DISCN = DISCN
Tradename = MONOPRIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 25069
Application Number = 19915
Date of Application = 16,, May, 1991
RX/OTC/DISCN = DISCN
Tradename = MONOPRIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 003
Tecode =
Rld = No
Strength = 20MG
----
Product id = 25070
Application Number = 19915
Date of Application = 28,, Mar, 1995
RX/OTC/DISCN = DISCN
Tradename = MONOPRIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 004
Tecode =
Rld = No
Strength = 40MG
----
Product id = 25072
Application Number = 20286
Date of Application = 30,, Nov, 1994
RX/OTC/DISCN = DISCN
Tradename = MONOPRIL-HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = No
Strength = 20MG;12.5MG
----
Product id = 25071
Application Number = 20286
Date of Application = 30,, Nov, 1994
RX/OTC/DISCN = DISCN
Tradename = MONOPRIL-HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21578
Application Number = 76139
Date of Application = 25,, Nov, 2003
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 21579
Application Number = 76139
Date of Application = 25,, Nov, 2003
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 21580
Application Number = 76139
Date of Application = 25,, Nov, 2003
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 40MG
----
Product id = 21572
Application Number = 76188
Date of Application = 8,, Oct, 2004
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 21574
Application Number = 76188
Date of Application = 8,, Oct, 2004
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG
----
Product id = 21576
Application Number = 76188
Date of Application = 8,, Oct, 2004
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 40MG
----
Product id = 21573
Application Number = 76483
Date of Application = 23,, Apr, 2004
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 21575
Application Number = 76483
Date of Application = 23,, Apr, 2004
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 21577
Application Number = 76483
Date of Application = 23,, Apr, 2004
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 21569
Application Number = 76580
Date of Application = 23,, Apr, 2004
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 21570
Application Number = 76580
Date of Application = 23,, Apr, 2004
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG
----
Product id = 21571
Application Number = 76580
Date of Application = 23,, Apr, 2004
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 003
Tecode =
Rld = No
Strength = 40MG
----
Product id = 21587
Application Number = 76608
Date of Application = 3,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21588
Application Number = 76608
Date of Application = 3,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 21557
Application Number = 76620
Date of Application = 15,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 21558
Application Number = 76620
Date of Application = 15,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 21559
Application Number = 76620
Date of Application = 15,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 21597
Application Number = 76739
Date of Application = 17,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21598
Application Number = 76739
Date of Application = 17,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 21560
Application Number = 76906
Date of Application = 17,, May, 2005
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 21561
Application Number = 76906
Date of Application = 17,, May, 2005
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 21562
Application Number = 76906
Date of Application = 17,, May, 2005
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 21601
Application Number = 76945
Date of Application = 5,, Jul, 2006
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21602
Application Number = 76945
Date of Application = 5,, Jul, 2006
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG;12.5MG
----
Product id = 21599
Application Number = 76961
Date of Application = 28,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21600
Application Number = 76961
Date of Application = 28,, Sep, 2005
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 21581
Application Number = 76987
Date of Application = 23,, Dec, 2004
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 21583
Application Number = 76987
Date of Application = 23,, Dec, 2004
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG
----
Product id = 21585
Application Number = 76987
Date of Application = 23,, Dec, 2004
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 40MG
----
Product id = 21603
Application Number = 77144
Date of Application = 16,, Aug, 2005
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21604
Application Number = 77144
Date of Application = 16,, Aug, 2005
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG;12.5MG
----
Product id = 21566
Application Number = 77222
Date of Application = 20,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 21567
Application Number = 77222
Date of Application = 20,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 21568
Application Number = 77222
Date of Application = 20,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 21582
Application Number = 77531
Date of Application = 31,, Aug, 2006
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG
----
Product id = 21584
Application Number = 77531
Date of Application = 31,, Aug, 2006
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG
----
Product id = 21586
Application Number = 77531
Date of Application = 31,, Aug, 2006
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 40MG
----
Product id = 21595
Application Number = 77705
Date of Application = 14,, Aug, 2006
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21596
Application Number = 77705
Date of Application = 14,, Aug, 2006
RX/OTC/DISCN = DISCN
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode =
Rld = No
Strength = 20MG;12.5MG
----
Product id = 21591
Application Number = 79025
Date of Application = 17,, Sep, 2010
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EMCURE PHARMS INDIA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21592
Application Number = 79025
Date of Application = 17,, Sep, 2010
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EMCURE PHARMS INDIA
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 20MG;12.5MG
----
Product id = 21589
Application Number = 79245
Date of Application = 9,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21590
Application Number = 79245
Date of Application = 9,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 21593
Application Number = 90228
Date of Application = 9,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG;12.5MG
----
Product id = 21594
Application Number = 90228
Date of Application = 9,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG;12.5MG
----
Product id = 21563
Application Number = 91163
Date of Application = 30,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 21564
Application Number = 91163
Date of Application = 30,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 21565
Application Number = 91163
Date of Application = 30,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = FOSINOPRIL SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 40MG
----