kanamycin-sulfate
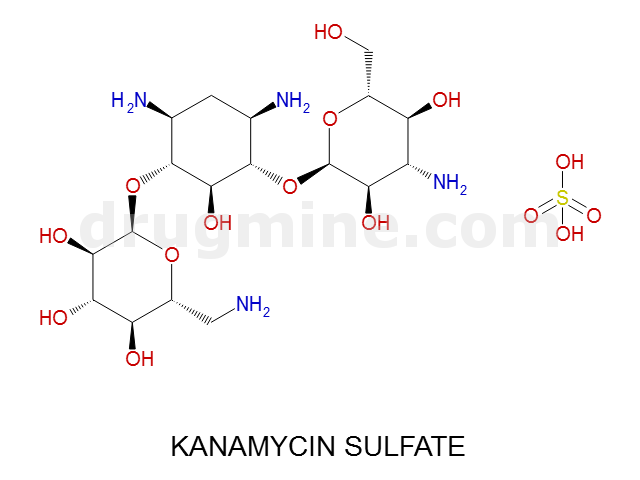

Name: KANAMYCIN SULFATE
ID :
MW: 484
Number of atoms: 33
Molecular_Formula: C18H36N4O11
Alogp: -7.144
Indication class : Antibacterial
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
kanamycin sulfate containing products summary
There are in total 36 different products containing the active ingredient kanamycin sulfate. From the 36 drug products, 34 have been discontinued.Product id = 2318
Application Number = 60516
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KANTREX
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 8584
Application Number = 61655
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KANTREX
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/2ML
----
Product id = 8578
Application Number = 61655
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KANTREX
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1GM BASE/3ML
----
Product id = 8581
Application Number = 61655
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KANTREX
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 75MG BASE/2ML
----
Product id = 8585
Application Number = 61901
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KANTREX
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/2ML
----
Product id = 8579
Application Number = 61901
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KANTREX
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1GM BASE/3ML
----
Product id = 8582
Application Number = 61901
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KANTREX
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 75MG BASE/2ML
----
Product id = 2319
Application Number = 61911
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KANTREX
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 8664
Application Number = 62170
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KLEBCIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = KING PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 75MG BASE/2ML
----
Product id = 8665
Application Number = 62170
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KLEBCIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = KING PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/2ML
----
Product id = 8663
Application Number = 62170
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KLEBCIL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = KING PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/3ML
----
Product id = 8558
Application Number = 62324
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 75MG BASE/2ML
----
Product id = 8559
Application Number = 62324
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/2ML
----
Product id = 8557
Application Number = 62324
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/3ML
----
Product id = 8566
Application Number = 62466
Date of Application = 30,, Sep, 1983
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = INTL MEDICATION
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/2ML
----
Product id = 8565
Application Number = 62466
Date of Application = 30,, Sep, 1983
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = INTL MEDICATION
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1GM BASE/3ML
----
Product id = 8561
Application Number = 62504
Date of Application = 5,, Apr, 1984
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 75MG BASE/2ML
----
Product id = 8562
Application Number = 62504
Date of Application = 5,, Apr, 1984
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/2ML
----
Product id = 8560
Application Number = 62504
Date of Application = 5,, Apr, 1984
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/3ML
----
Product id = 8577
Application Number = 62520
Date of Application = 9,, May, 1985
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/3ML
----
Product id = 8583
Application Number = 62564
Date of Application = 21,, Sep, 1984
RX/OTC/DISCN = DISCN
Tradename = KANTREX
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 75MG BASE/2ML
----
Product id = 8586
Application Number = 62564
Date of Application = 21,, Sep, 1984
RX/OTC/DISCN = DISCN
Tradename = KANTREX
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/2ML
----
Product id = 8580
Application Number = 62564
Date of Application = 21,, Sep, 1984
RX/OTC/DISCN = DISCN
Tradename = KANTREX
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/3ML
----
Product id = 8575
Application Number = 62605
Date of Application = 26,, Feb, 1986
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SOLOPAK
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/2ML
----
Product id = 8573
Application Number = 62605
Date of Application = 26,, Feb, 1986
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SOLOPAK
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1GM BASE/3ML
----
Product id = 8574
Application Number = 62605
Date of Application = 26,, Feb, 1986
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SOLOPAK
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 75MG BASE/2ML
----
Product id = 8571
Application Number = 62668
Date of Application = 7,, May, 1987
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = PHARMAFAIR
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 75MG BASE/2ML
----
Product id = 8570
Application Number = 62669
Date of Application = 7,, May, 1987
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = PHARMAFAIR
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1GM BASE/3ML
----
Product id = 8572
Application Number = 62672
Date of Application = 7,, May, 1987
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = PHARMAFAIR
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/2ML
----
Product id = 2320
Application Number = 62726
Date of Application = 6,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = KANTREX
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 8568
Application Number = 63021
Date of Application = 31,, Jul, 1992
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LOCH
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 75MG BASE/2ML
----
Product id = 8569
Application Number = 63022
Date of Application = 31,, Jul, 1992
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LOCH
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/2ML
----
Product id = 8567
Application Number = 63025
Date of Application = 31,, Jul, 1992
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LOCH
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1GM BASE/3ML
----
Product id = 8576
Application Number = 63092
Date of Application = 11,, Oct, 1989
RX/OTC/DISCN = DISCN
Tradename = KANAMYCIN SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1GM BASE/3ML
----
Product id = 8564
Application Number = 65111
Date of Application = 17,, Dec, 2002
RX/OTC/DISCN = RX
Tradename = KANAMYCIN SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/2ML
----
Product id = 8563
Application Number = 65111
Date of Application = 17,, Dec, 2002
RX/OTC/DISCN = RX
Tradename = KANAMYCIN SULFATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 1GM BASE/3ML
----