lithium-carbonate
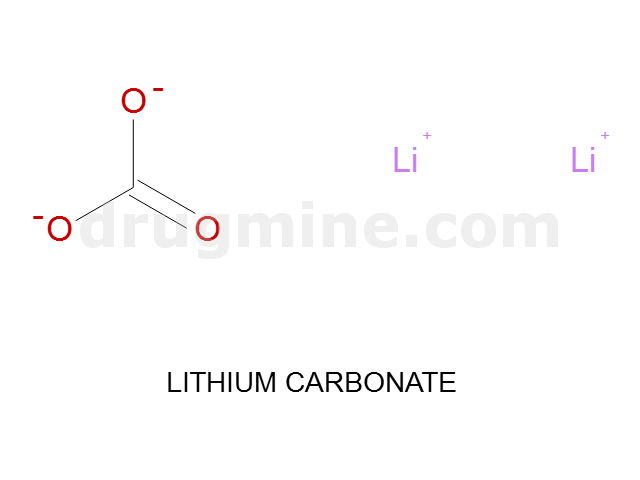

Name: LITHIUM CARBONATE
ID :
MW: 60
Number of atoms: 4
Molecular_Formula: CO3
Alogp: -1.965
Indication class : Antimanic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
lithium carbonate containing products summary
There are in total 43 different products containing the active ingredient lithium carbonate. From the 43 drug products, 20 have been discontinued.Product id = 2386
Application Number = 16782
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LITHONATE
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 23832
Application Number = 16834
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LITHIUM CARBONATE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 1863
Application Number = 16860
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ESKALITH
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = NOVEN THERAP
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 23836
Application Number = 16980
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = LITHOTABS
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SOLVAY
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 2382
Application Number = 17812
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 2381
Application Number = 17812
Date of Application = 28,, Jan, 1987
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ROXANE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 2383
Application Number = 17812
Date of Application = 28,, Jan, 1987
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ROXANE
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 600MG
----
Product id = 20980
Application Number = 17971
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ESKALITH
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JDS PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 16146
Application Number = 18027
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = LITHOBID
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 300MG
----
Product id = 15910
Application Number = 18152
Date of Application = 29,, Mar, 1982
RX/OTC/DISCN = DISCN
Tradename = ESKALITH CR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = JDS PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 450MG
----
Product id = 23833
Application Number = 18558
Date of Application = 29,, Jan, 1982
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 300MG
----
Product id = 23830
Application Number = 18833
Date of Application = 18,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = LITHANE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BAYER PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 2385
Application Number = 70407
Date of Application = 19,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 2384
Application Number = 72542
Date of Application = 1,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 2365
Application Number = 76121
Date of Application = 27,, Sep, 2001
RX/OTC/DISCN = DISCN
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ABLE
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 16137
Application Number = 76170
Date of Application = 10,, Jun, 2002
RX/OTC/DISCN = DISCN
Tradename = LITHIUM CARBONATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 2379
Application Number = 76243
Date of Application = 27,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 2378
Application Number = 76243
Date of Application = 24,, Feb, 2003
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 16138
Application Number = 76366
Date of Application = 21,, Aug, 2003
RX/OTC/DISCN = DISCN
Tradename = LITHIUM CARBONATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 450MG
----
Product id = 16136
Application Number = 76382
Date of Application = 21,, Apr, 2003
RX/OTC/DISCN = DISCN
Tradename = LITHIUM CARBONATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ABLE
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 16141
Application Number = 76490
Date of Application = 17,, Jun, 2003
RX/OTC/DISCN = DISCN
Tradename = LITHIUM CARBONATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 450MG
----
Product id = 16145
Application Number = 76691
Date of Application = 5,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 450MG
----
Product id = 2371
Application Number = 76795
Date of Application = 22,, Nov, 2004
RX/OTC/DISCN = DISCN
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 2364
Application Number = 76823
Date of Application = 29,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ABLE
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG
----
Product id = 2366
Application Number = 76823
Date of Application = 29,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ABLE
ProductNo = 002
Tecode =
Rld = No
Strength = 300MG
----
Product id = 2367
Application Number = 76823
Date of Application = 29,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ABLE
ProductNo = 003
Tecode =
Rld = No
Strength = 600MG
----
Product id = 16144
Application Number = 76832
Date of Application = 28,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 23831
Application Number = 78715
Date of Application = 28,, Dec, 2010
RX/OTC/DISCN = DISCN
Tradename = LITHIUM CARBONATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA INTL PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 300MG
----
Product id = 2380
Application Number = 78763
Date of Application = 15,, Apr, 2008
RX/OTC/DISCN = DISCN
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = HIKMA PHARMS LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 600MG
----
Product id = 2372
Application Number = 79139
Date of Application = 3,, Feb, 2009
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 2373
Application Number = 79139
Date of Application = 3,, Feb, 2009
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 2374
Application Number = 79139
Date of Application = 3,, Feb, 2009
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 600MG
----
Product id = 2368
Application Number = 79159
Date of Application = 12,, Jan, 2009
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 2369
Application Number = 79159
Date of Application = 12,, Jan, 2009
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 2370
Application Number = 79159
Date of Application = 12,, Jan, 2009
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ALEMBIC LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 600MG
----
Product id = 2375
Application Number = 90702
Date of Application = 25,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = HETERO LABS LTD III
ProductNo = 001
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 2376
Application Number = 90702
Date of Application = 25,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = HETERO LABS LTD III
ProductNo = 002
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 2377
Application Number = 90702
Date of Application = 25,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = HETERO LABS LTD III
ProductNo = 003
Tecode = AB
Rld = No
Strength = 600MG
----
Product id = 23834
Application Number = 91027
Date of Application = 24,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 16139
Application Number = 91544
Date of Application = 27,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 300MG
----
Product id = 16140
Application Number = 91616
Date of Application = 14,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 450MG
----
Product id = 16143
Application Number = 202219
Date of Application = 8,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 450MG
----
Product id = 16142
Application Number = 202288
Date of Application = 29,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = LITHIUM CARBONATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 300MG
----