loperamide-hydrochloride
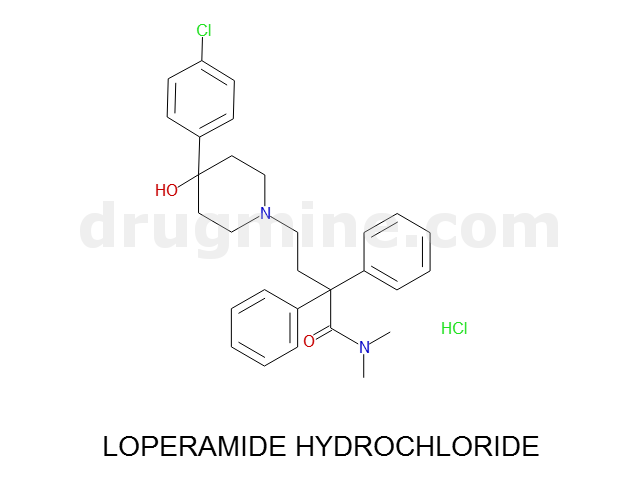
Name: LOPERAMIDE HYDROCHLORIDE
ID :
MW: 477
Number of atoms: 34
Molecular_Formula: C29H33ClN2O2
Alogp: 4.635
Indication class : Antiperistaltic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
loperamide hydrochloride containing products summary
There are in total 33 different products containing the active ingredient loperamide hydrochloride. From the 33 drug products, 11 have been discontinued.Product id = 2244
Application Number = 17690
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = IMODIUM
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = MCNEIL PED
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 2243
Application Number = 17694
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = IMODIUM
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = MCNEIL CONS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 2MG
----
Product id = 13857
Application Number = 19037
Date of Application = 31,, Jul, 1984
RX/OTC/DISCN = DISCN
Tradename = IMODIUM
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/5ML
----
Product id = 13858
Application Number = 19487
Date of Application = 1,, Mar, 1988
RX/OTC/DISCN = OTC
Tradename = IMODIUM A-D
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = MCNEIL CONS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 1MG/5ML
----
Product id = 14723
Application Number = 19487
Date of Application = 8,, Jul, 2004
RX/OTC/DISCN = OTC
Tradename = IMODIUM A-D
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = MCNEIL CONS
ProductNo = 002
Tecode =
Rld = Yes
Strength = 1MG/7.5ML
----
Product id = 22896
Application Number = 19860
Date of Application = 22,, Nov, 1989
RX/OTC/DISCN = OTC
Tradename = IMODIUM A-D
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MCNEIL CONS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 2MG
----
Product id = 15267
Application Number = 20448
Date of Application = 24,, Jul, 1997
RX/OTC/DISCN = OTC
Tradename = IMODIUM A-D EZ CHEWS
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = MCNEIL
ProductNo = 001
Tecode =
Rld = Yes
Strength = 2MG
----
Product id = 15268
Application Number = 20606
Date of Application = 26,, Jun, 1996
RX/OTC/DISCN = OTC
Tradename = IMODIUM MULTI-SYMPTOM RELIEF
Route/format = ORAL / TABLET, CHEWABLE
Application Type = N
Applicant Name = MCNEIL
ProductNo = 001
Tecode =
Rld = Yes
Strength = 2MG;125MG
----
Product id = 22897
Application Number = 21140
Date of Application = 30,, Nov, 2000
RX/OTC/DISCN = OTC
Tradename = IMODIUM MULTI-SYMPTOM RELIEF
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MCNEIL CONS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 2MG;125MG
----
Product id = 2389
Application Number = 21855
Date of Application = 4,, Aug, 2005
RX/OTC/DISCN = OTC
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = BANNER LIFE SCIENCES
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG
----
Product id = 2390
Application Number = 21855
Date of Application = 4,, Aug, 2005
RX/OTC/DISCN = OTC
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = BANNER LIFE SCIENCES
ProductNo = 002
Tecode =
Rld = Yes
Strength = 2MG
----
Product id = 2391
Application Number = 72741
Date of Application = 18,, Sep, 1991
RX/OTC/DISCN = RX
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 2393
Application Number = 72993
Date of Application = 28,, Aug, 1992
RX/OTC/DISCN = DISCN
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 13916
Application Number = 73062
Date of Application = 28,, May, 1993
RX/OTC/DISCN = DISCN
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/5ML
----
Product id = 13914
Application Number = 73079
Date of Application = 30,, Apr, 1992
RX/OTC/DISCN = OTC
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/5ML
----
Product id = 2392
Application Number = 73080
Date of Application = 27,, Nov, 1991
RX/OTC/DISCN = DISCN
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 2394
Application Number = 73122
Date of Application = 30,, Aug, 1991
RX/OTC/DISCN = DISCN
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 13910
Application Number = 73187
Date of Application = 15,, Sep, 1992
RX/OTC/DISCN = DISCN
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ALPHARMA US PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/5ML
----
Product id = 2395
Application Number = 73192
Date of Application = 30,, Apr, 1992
RX/OTC/DISCN = RX
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 13913
Application Number = 73243
Date of Application = 21,, Jan, 1992
RX/OTC/DISCN = OTC
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/5ML
----
Product id = 23852
Application Number = 73254
Date of Application = 30,, Jul, 1993
RX/OTC/DISCN = OTC
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CONTRACT PHARMACAL
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 13915
Application Number = 73478
Date of Application = 23,, Jun, 1995
RX/OTC/DISCN = DISCN
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/5ML
----
Product id = 23851
Application Number = 73528
Date of Application = 30,, Nov, 1993
RX/OTC/DISCN = DISCN
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ABLE
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 23854
Application Number = 74091
Date of Application = 10,, Dec, 1992
RX/OTC/DISCN = OTC
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = OHM LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 23855
Application Number = 74194
Date of Application = 30,, Oct, 1992
RX/OTC/DISCN = DISCN
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 13912
Application Number = 74352
Date of Application = 17,, Nov, 1995
RX/OTC/DISCN = OTC
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = HI TECH PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/5ML
----
Product id = 13917
Application Number = 74730
Date of Application = 28,, Aug, 1997
RX/OTC/DISCN = OTC
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/5ML
----
Product id = 13911
Application Number = 74991
Date of Application = 29,, Dec, 1997
RX/OTC/DISCN = DISCN
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = DURAMED PHARMS BARR
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/5ML
----
Product id = 23856
Application Number = 75232
Date of Application = 6,, Jan, 2000
RX/OTC/DISCN = OTC
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 15300
Application Number = 76029
Date of Application = 30,, Aug, 2002
RX/OTC/DISCN = OTC
Tradename = LOPERAMIDE HYDROCHLORIDE AND SIMETHICONE
Route/format = ORAL / TABLET, CHEWABLE
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG;125MG
----
Product id = 23853
Application Number = 76497
Date of Application = 10,, Jun, 2003
RX/OTC/DISCN = OTC
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LNK
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 23857
Application Number = 77500
Date of Application = 6,, Sep, 2006
RX/OTC/DISCN = OTC
Tradename = LOPERAMIDE HYDROCHLORIDE AND SIMETHICONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG;125MG
----
Product id = 14729
Application Number = 91292
Date of Application = 20,, May, 2011
RX/OTC/DISCN = OTC
Tradename = LOPERAMIDE HYDROCHLORIDE
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = PERRIGO R AND D
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/7.5ML
----