mexiletine-hydrochloride

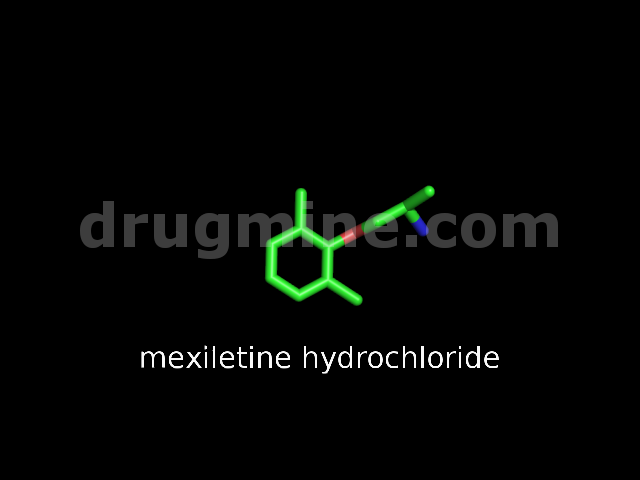
Name: MEXILETINE HYDROCHLORIDE
ID :
MW: 179
Number of atoms: 13
Molecular_Formula: C11H17NO
Alogp: 2.334
Indication class : Cardiac Depressant (anti-arrhythmic)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
mexiletine hydrochloride containing products summary
There are in total 15 different products containing the active ingredient mexiletine hydrochloride. From the 15 drug products, 12 have been discontinued.Product id = 2501
Application Number = 18873
Date of Application = 30,, Dec, 1985
RX/OTC/DISCN = DISCN
Tradename = MEXITIL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 002
Tecode =
Rld = No
Strength = 150MG
----
Product id = 2502
Application Number = 18873
Date of Application = 30,, Dec, 1985
RX/OTC/DISCN = DISCN
Tradename = MEXITIL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 003
Tecode =
Rld = No
Strength = 200MG
----
Product id = 2503
Application Number = 18873
Date of Application = 30,, Dec, 1985
RX/OTC/DISCN = DISCN
Tradename = MEXITIL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 004
Tecode =
Rld = No
Strength = 250MG
----
Product id = 2492
Application Number = 74377
Date of Application = 16,, May, 1995
RX/OTC/DISCN = RX
Tradename = MEXILETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG
----
Product id = 2493
Application Number = 74377
Date of Application = 16,, May, 1995
RX/OTC/DISCN = RX
Tradename = MEXILETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG
----
Product id = 2494
Application Number = 74377
Date of Application = 16,, May, 1995
RX/OTC/DISCN = RX
Tradename = MEXILETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode =
Rld = Yes
Strength = 250MG
----
Product id = 2489
Application Number = 74450
Date of Application = 16,, May, 1996
RX/OTC/DISCN = DISCN
Tradename = MEXILETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG
----
Product id = 2490
Application Number = 74450
Date of Application = 16,, May, 1996
RX/OTC/DISCN = DISCN
Tradename = MEXILETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG
----
Product id = 2491
Application Number = 74450
Date of Application = 16,, May, 1996
RX/OTC/DISCN = DISCN
Tradename = MEXILETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 250MG
----
Product id = 2495
Application Number = 74711
Date of Application = 26,, Feb, 1997
RX/OTC/DISCN = DISCN
Tradename = MEXILETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG
----
Product id = 2497
Application Number = 74711
Date of Application = 26,, Feb, 1997
RX/OTC/DISCN = DISCN
Tradename = MEXILETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG
----
Product id = 2499
Application Number = 74711
Date of Application = 26,, Feb, 1997
RX/OTC/DISCN = DISCN
Tradename = MEXILETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 250MG
----
Product id = 2496
Application Number = 74865
Date of Application = 13,, Apr, 1998
RX/OTC/DISCN = DISCN
Tradename = MEXILETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 150MG
----
Product id = 2498
Application Number = 74865
Date of Application = 13,, Apr, 1998
RX/OTC/DISCN = DISCN
Tradename = MEXILETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 200MG
----
Product id = 2500
Application Number = 74865
Date of Application = 13,, Apr, 1998
RX/OTC/DISCN = DISCN
Tradename = MEXILETINE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 250MG
----