mitoxantrone-hydrochloride
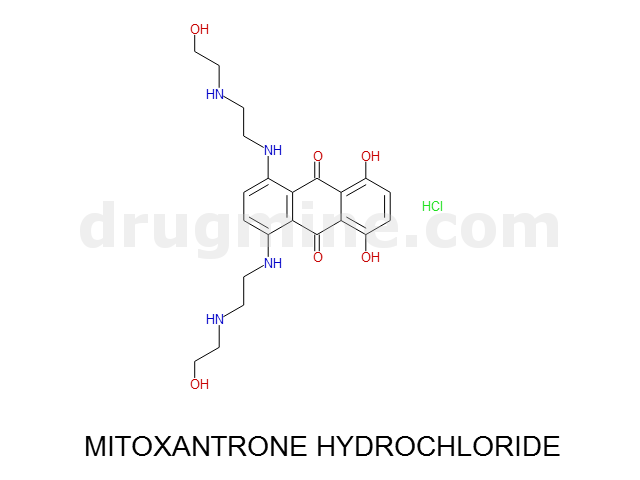


Name: MITOXANTRONE HYDROCHLORIDE
ID :
MW: 444
Number of atoms: 32
Molecular_Formula: C22H28N4O6
Alogp: 6.8e-002
Indication class : Antineoplastic
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
mitoxantrone hydrochloride containing products summary
There are in total 21 different products containing the active ingredient mitoxantrone hydrochloride. From the 21 drug products, 6 have been discontinued.Product id = 9720
Application Number = 19297
Date of Application = 23,, Dec, 1987
RX/OTC/DISCN = DISCN
Tradename = NOVANTRONE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = EMD SERONO
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 20MG BASE/10ML (EQ 2MG BASE/ML)
----
Product id = 9721
Application Number = 19297
Date of Application = 23,, Dec, 1987
RX/OTC/DISCN = DISCN
Tradename = NOVANTRONE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = EMD SERONO
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 25MG BASE/12.5ML (EQ 2MG BASE/ML)
----
Product id = 9722
Application Number = 19297
Date of Application = 23,, Dec, 1987
RX/OTC/DISCN = DISCN
Tradename = NOVANTRONE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = EMD SERONO
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 30MG BASE/15ML (EQ 2MG BASE/ML)
----
Product id = 9417
Application Number = 76611
Date of Application = 11,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = MITOXANTRONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EUROHLTH INTL SARL
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 20MG BASE/10ML (EQ 2MG BASE/ML)
----
Product id = 9418
Application Number = 76611
Date of Application = 11,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = MITOXANTRONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EUROHLTH INTL SARL
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 25MG BASE/12.5ML (EQ 2MG BASE/ML)
----
Product id = 9419
Application Number = 76611
Date of Application = 11,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = MITOXANTRONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EUROHLTH INTL SARL
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 30MG BASE/15ML (EQ 2MG BASE/ML)
----
Product id = 9426
Application Number = 76871
Date of Application = 11,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = MITOXANTRONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 20MG BASE/10ML (EQ 2MG BASE/ML)
----
Product id = 9427
Application Number = 76871
Date of Application = 11,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = MITOXANTRONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 25MG BASE/12.5ML (EQ 2MG BASE/ML)
----
Product id = 9428
Application Number = 76871
Date of Application = 11,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = MITOXANTRONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = EQ 30MG BASE/15ML (EQ 2MG BASE/ML)
----
Product id = 9432
Application Number = 77356
Date of Application = 11,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = MITOXANTRONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 20MG BASE/10ML (EQ 2MG BASE/ML)
----
Product id = 9433
Application Number = 77356
Date of Application = 11,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = MITOXANTRONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 25MG BASE/12.5ML (EQ 2MG BASE/ML)
----
Product id = 9434
Application Number = 77356
Date of Application = 11,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = MITOXANTRONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 30MG BASE/15ML (EQ 2MG BASE/ML)
----
Product id = 9423
Application Number = 77496
Date of Application = 11,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = MITOXANTRONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 20MG BASE/10ML (EQ 2MG BASE/ML)
----
Product id = 9424
Application Number = 77496
Date of Application = 11,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = MITOXANTRONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 25MG BASE/12.5ML (EQ 2MG BASE/ML)
----
Product id = 9425
Application Number = 77496
Date of Application = 11,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = MITOXANTRONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 30MG BASE/15ML (EQ 2MG BASE/ML)
----
Product id = 9420
Application Number = 78606
Date of Application = 14,, May, 2008
RX/OTC/DISCN = DISCN
Tradename = MITOXANTRONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI ONCOL
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 20MG BASE/10ML (EQ 2MG BASE/ML)
----
Product id = 9421
Application Number = 78606
Date of Application = 14,, May, 2008
RX/OTC/DISCN = DISCN
Tradename = MITOXANTRONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI ONCOL
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 25MG BASE/12.5ML (EQ 2MG BASE/ML)
----
Product id = 9422
Application Number = 78606
Date of Application = 14,, May, 2008
RX/OTC/DISCN = DISCN
Tradename = MITOXANTRONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI ONCOL
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 30MG BASE/15ML (EQ 2MG BASE/ML)
----
Product id = 9429
Application Number = 78980
Date of Application = 13,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = MITOXANTRONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MYLAN INSTITUTIONAL
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 20MG BASE/10ML (EQ 2MG BASE/ML)
----
Product id = 9430
Application Number = 78980
Date of Application = 13,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = MITOXANTRONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MYLAN INSTITUTIONAL
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 30MG BASE/15ML (EQ 2MG BASE/ML)
----
Product id = 9431
Application Number = 201014
Date of Application = 11,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = MITOXANTRONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ONCO THERAPIES LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 20MG BASE/10ML (EQ 2MG BASE/ML)
----