naloxone-hydrochloride
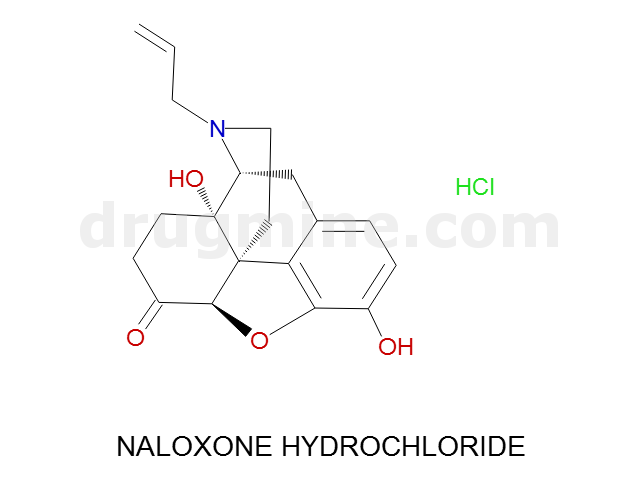

Name: NALOXONE HYDROCHLORIDE
ID :
MW: 327
Number of atoms: 24
Molecular_Formula: C19H21NO4
Alogp: 1.453
Indication class : Antagonist (to narcotics)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
naloxone hydrochloride containing products summary
There are in total 83 different products containing the active ingredient naloxone hydrochloride. From the 83 drug products, 53 have been discontinued.Product id = 9602
Application Number = 16636
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NARCAN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ENDO PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 9601
Application Number = 16636
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = NARCAN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ENDO PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.02MG/ML **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 9603
Application Number = 16636
Date of Application = 14,, Jun, 1982
RX/OTC/DISCN = DISCN
Tradename = NARCAN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ENDO PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 1MG/ML **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 28446
Application Number = 18733
Date of Application = 16,, Dec, 1982
RX/OTC/DISCN = DISCN
Tradename = TALWIN NX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SANOFI AVENTIS US
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.5MG BASE;EQ 50MG BASE
----
Product id = 30213
Application Number = 20733
Date of Application = 8,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = SUBOXONE
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = RECKITT BENCKISER
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE;EQ 0.5MG BASE
----
Product id = 30214
Application Number = 20733
Date of Application = 8,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = SUBOXONE
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = RECKITT BENCKISER
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 8MG BASE;EQ 2MG BASE
----
Product id = 4801
Application Number = 22410
Date of Application = 30,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = SUBOXONE
Route/format = SUBLINGUAL / FILM
Application Type = N
Applicant Name = RECKITT BENCKISER
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE;EQ 0.5MG BASE
----
Product id = 4803
Application Number = 22410
Date of Application = 30,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = SUBOXONE
Route/format = SUBLINGUAL / FILM
Application Type = N
Applicant Name = RECKITT BENCKISER
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 8MG BASE;EQ 2MG BASE
----
Product id = 4802
Application Number = 22410
Date of Application = 10,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = SUBOXONE
Route/format = SUBLINGUAL / FILM
Application Type = N
Applicant Name = RECKITT BENCKISER
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 4MG BASE;EQ 1MG BASE
----
Product id = 4804
Application Number = 22410
Date of Application = 10,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = SUBOXONE
Route/format = SUBLINGUAL / FILM
Application Type = N
Applicant Name = RECKITT BENCKISER
ProductNo = 004
Tecode =
Rld = Yes
Strength = EQ 12MG BASE;EQ 3MG BASE
----
Product id = 9566
Application Number = 70171
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.02MG/ML
----
Product id = 9569
Application Number = 70172
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = RX
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 0.4MG/ML
----
Product id = 9541
Application Number = 70188
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = NALOXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WYETH AYERST
ProductNo = 001
Tecode =
Rld = No
Strength = 0.02MG/ML
----
Product id = 9542
Application Number = 70189
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = NALOXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WYETH AYERST
ProductNo = 001
Tecode =
Rld = No
Strength = 0.02MG/ML
----
Product id = 9543
Application Number = 70190
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = NALOXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WYETH AYERST
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9544
Application Number = 70191
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = NALOXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WYETH AYERST
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9567
Application Number = 70252
Date of Application = 16,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.02MG/ML
----
Product id = 9568
Application Number = 70253
Date of Application = 16,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.02MG/ML
----
Product id = 9570
Application Number = 70254
Date of Application = 7,, Jan, 1987
RX/OTC/DISCN = RX
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 0.4MG/ML
----
Product id = 9571
Application Number = 70255
Date of Application = 7,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9572
Application Number = 70256
Date of Application = 7,, Jan, 1987
RX/OTC/DISCN = RX
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 0.4MG/ML
----
Product id = 9573
Application Number = 70257
Date of Application = 7,, Jan, 1987
RX/OTC/DISCN = RX
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 0.4MG/ML
----
Product id = 9538
Application Number = 70298
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = NALOXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9539
Application Number = 70299
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = NALOXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9574
Application Number = 70417
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = INTL MEDICATION
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9540
Application Number = 70496
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = NALOXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9575
Application Number = 70639
Date of Application = 24,, Sep, 1986
RX/OTC/DISCN = RX
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = INTL MEDICATION
ProductNo = 001
Tecode = AP
Rld = No
Strength = 0.4MG/ML
----
Product id = 9545
Application Number = 70648
Date of Application = 17,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.02MG/ML
----
Product id = 9547
Application Number = 70649
Date of Application = 17,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9546
Application Number = 70661
Date of Application = 17,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.02MG/ML
----
Product id = 9598
Application Number = 71083
Date of Application = 28,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = NARCAN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9599
Application Number = 71084
Date of Application = 28,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = NARCAN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 9562
Application Number = 71272
Date of Application = 24,, May, 1988
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = 0.02MG/ML
----
Product id = 9563
Application Number = 71273
Date of Application = 24,, May, 1988
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 9564
Application Number = 71274
Date of Application = 24,, May, 1988
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 9565
Application Number = 71287
Date of Application = 24,, May, 1988
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 9600
Application Number = 71311
Date of Application = 28,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = NARCAN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 9585
Application Number = 71339
Date of Application = 18,, Nov, 1987
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9548
Application Number = 71604
Date of Application = 16,, Dec, 1988
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 9580
Application Number = 71671
Date of Application = 17,, Nov, 1987
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SMITH AND NEPHEW
ProductNo = 001
Tecode =
Rld = No
Strength = 0.02MG/ML
----
Product id = 9583
Application Number = 71672
Date of Application = 17,, Nov, 1987
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SOLOPAK
ProductNo = 001
Tecode =
Rld = No
Strength = 0.02MG/ML
----
Product id = 9581
Application Number = 71681
Date of Application = 17,, Nov, 1987
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SMITH AND NEPHEW
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9582
Application Number = 71682
Date of Application = 17,, Nov, 1987
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SMITH AND NEPHEW
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9584
Application Number = 71683
Date of Application = 17,, Nov, 1987
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SOLOPAK
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9578
Application Number = 71811
Date of Application = 19,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MARSAM PHARMS LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9576
Application Number = 72076
Date of Application = 24,, Mar, 1988
RX/OTC/DISCN = RX
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = INTL MEDICATION
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 1MG/ML
----
Product id = 9549
Application Number = 72081
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.02MG/ML
----
Product id = 9550
Application Number = 72082
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.02MG/ML
----
Product id = 9551
Application Number = 72083
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.02MG/ML
----
Product id = 9552
Application Number = 72084
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.02MG/ML
----
Product id = 9553
Application Number = 72085
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.02MG/ML
----
Product id = 9554
Application Number = 72086
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9555
Application Number = 72087
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9556
Application Number = 72088
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9557
Application Number = 72089
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9558
Application Number = 72090
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.4MG/ML
----
Product id = 9559
Application Number = 72091
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 9560
Application Number = 72092
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 9561
Application Number = 72093
Date of Application = 11,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 9577
Application Number = 72115
Date of Application = 27,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = INTL MEDICATION
ProductNo = 001
Tecode =
Rld = No
Strength = 1MG/ML
----
Product id = 25182
Application Number = 74736
Date of Application = 21,, Jan, 1997
RX/OTC/DISCN = RX
Tradename = NALOXONE HYDROCHLORIDE AND PENTAZOCINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 0.5MG BASE;EQ 50MG BASE
----
Product id = 25181
Application Number = 75523
Date of Application = 17,, Mar, 2000
RX/OTC/DISCN = RX
Tradename = NALOXONE HYDROCHLORIDE AND PENTAZOCINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE;EQ 50MG BASE
----
Product id = 25180
Application Number = 75735
Date of Application = 11,, Jul, 2001
RX/OTC/DISCN = RX
Tradename = NALOXONE HYDROCHLORIDE AND PENTAZOCINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GAVIS PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.5MG BASE;EQ 50MG BASE
----
Product id = 30160
Application Number = 91149
Date of Application = 8,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE;EQ 0.5MG BASE
----
Product id = 30161
Application Number = 91149
Date of Application = 8,, Sep, 2014
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE;EQ 2MG BASE
----
Product id = 30155
Application Number = 91422
Date of Application = 22,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE;EQ 0.5MG BASE
----
Product id = 30156
Application Number = 91422
Date of Application = 22,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = EQ 8MG BASE;EQ 2MG BASE
----
Product id = 30162
Application Number = 203136
Date of Application = 22,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE;EQ 0.5MG BASE
----
Product id = 30157
Application Number = 203136
Date of Application = 22,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE;EQ 2MG BASE
----
Product id = 30158
Application Number = 203326
Date of Application = 27,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2MG BASE;EQ 0.5MG BASE
----
Product id = 30159
Application Number = 203326
Date of Application = 27,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = BUPRENORPHINE HYDROCHLORIDE AND NALOXONE HYDROCHLORIDE
Route/format = SUBLINGUAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 8MG BASE;EQ 2MG BASE
----
Product id = 30218
Application Number = 204242
Date of Application = 3,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = ZUBSOLV
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = OREXO AB
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1.4MG BASE;EQ 0.36MG BASE
----
Product id = 30219
Application Number = 204242
Date of Application = 3,, Jul, 2013
RX/OTC/DISCN = RX
Tradename = ZUBSOLV
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = OREXO AB
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 5.7MG BASE;EQ 1.4MG BASE
----
Product id = 30220
Application Number = 204242
Date of Application = 11,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = ZUBSOLV
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = OREXO AB
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 8.6MG BASE;EQ 2.1MG BASE
----
Product id = 30221
Application Number = 204242
Date of Application = 11,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = ZUBSOLV
Route/format = SUBLINGUAL / TABLET
Application Type = N
Applicant Name = OREXO AB
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 11.4MG BASE;EQ 2.9MG BASE
----
Product id = 9579
Application Number = 204997
Date of Application = 6,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = NALOXONE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MYLAN INSTITUTIONAL
ProductNo = 001
Tecode = AP
Rld = No
Strength = 0.4MG/ML
----
Product id = 4790
Application Number = 205637
Date of Application = 6,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = BUNAVAIL
Route/format = BUCCAL / FILM
Application Type = N
Applicant Name = BIODELIVERY SCI INTL
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2.1MG BASE;EQ 0.3MG BASE
----
Product id = 4791
Application Number = 205637
Date of Application = 6,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = BUNAVAIL
Route/format = BUCCAL / FILM
Application Type = N
Applicant Name = BIODELIVERY SCI INTL
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 4.2MG BASE;EQ 0.7MG BASE
----
Product id = 4792
Application Number = 205637
Date of Application = 6,, Jun, 2014
RX/OTC/DISCN = RX
Tradename = BUNAVAIL
Route/format = BUCCAL / FILM
Application Type = N
Applicant Name = BIODELIVERY SCI INTL
ProductNo = 003
Tecode =
Rld = Yes
Strength = EQ 6.3MG BASE;EQ 1MG BASE
----
Product id = 16655
Application Number = 205777
Date of Application = 23,, Jul, 2014
RX/OTC/DISCN = DISCN
Tradename = TARGINIQ
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = PURDUE PHARMA LP
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG;10MG
----
Product id = 16656
Application Number = 205777
Date of Application = 23,, Jul, 2014
RX/OTC/DISCN = DISCN
Tradename = TARGINIQ
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = PURDUE PHARMA LP
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG;20MG
----
Product id = 16657
Application Number = 205777
Date of Application = 23,, Jul, 2014
RX/OTC/DISCN = DISCN
Tradename = TARGINIQ
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = PURDUE PHARMA LP
ProductNo = 003
Tecode =
Rld = No
Strength = 20MG;40MG
----
Product id = 13467
Application Number = 205787
Date of Application = 3,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = EVZIO
Route/format = INTRAMUSCULAR, SUBCUTANEOUS / SOLUTION
Application Type = N
Applicant Name = KALEO INC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.4MG/0.4ML
----