nefazodone-hydrochloride
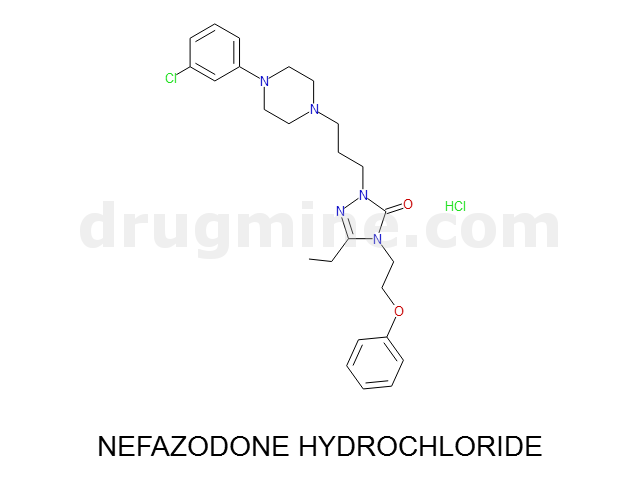

Name: NEFAZODONE HYDROCHLORIDE
ID :
MW: 470
Number of atoms: 33
Molecular_Formula: C25H32ClN5O2
Alogp: 4.415
Indication class : Antidepressant
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
nefazodone hydrochloride containing products summary
There are in total 49 different products containing the active ingredient nefazodone hydrochloride. From the 49 drug products, 34 have been discontinued.Product id = 27981
Application Number = 20152
Date of Application = 22,, Dec, 1994
RX/OTC/DISCN = DISCN
Tradename = SERZONE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 27982
Application Number = 20152
Date of Application = 22,, Dec, 1994
RX/OTC/DISCN = DISCN
Tradename = SERZONE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 27983
Application Number = 20152
Date of Application = 22,, Dec, 1994
RX/OTC/DISCN = DISCN
Tradename = SERZONE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG
----
Product id = 27984
Application Number = 20152
Date of Application = 22,, Dec, 1994
RX/OTC/DISCN = DISCN
Tradename = SERZONE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 004
Tecode =
Rld = No
Strength = 200MG
----
Product id = 27985
Application Number = 20152
Date of Application = 22,, Dec, 1994
RX/OTC/DISCN = DISCN
Tradename = SERZONE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 005
Tecode =
Rld = No
Strength = 250MG
----
Product id = 27986
Application Number = 20152
Date of Application = 22,, Dec, 1994
RX/OTC/DISCN = DISCN
Tradename = SERZONE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 006
Tecode =
Rld = No
Strength = 300MG
----
Product id = 25350
Application Number = 75763
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 25351
Application Number = 75763
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 25352
Application Number = 75763
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG
----
Product id = 25353
Application Number = 75763
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = 200MG
----
Product id = 25354
Application Number = 75763
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 005
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25379
Application Number = 76037
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 25380
Application Number = 76037
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 25381
Application Number = 76037
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 25382
Application Number = 76037
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 004
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 25383
Application Number = 76037
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 005
Tecode = AB
Rld = Yes
Strength = 250MG
----
Product id = 25369
Application Number = 76072
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 25371
Application Number = 76072
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 25373
Application Number = 76072
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG
----
Product id = 25375
Application Number = 76072
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode =
Rld = No
Strength = 200MG
----
Product id = 25377
Application Number = 76072
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 005
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25384
Application Number = 76073
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 25385
Application Number = 76073
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG
----
Product id = 25386
Application Number = 76073
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 004
Tecode =
Rld = No
Strength = 200MG
----
Product id = 25387
Application Number = 76073
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 005
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25355
Application Number = 76129
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 25356
Application Number = 76129
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG
----
Product id = 25357
Application Number = 76129
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode =
Rld = No
Strength = 200MG
----
Product id = 25358
Application Number = 76129
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 005
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25364
Application Number = 76196
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 25365
Application Number = 76196
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 25366
Application Number = 76196
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG
----
Product id = 25367
Application Number = 76196
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 004
Tecode =
Rld = No
Strength = 200MG
----
Product id = 25368
Application Number = 76196
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 005
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25370
Application Number = 76302
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG
----
Product id = 25372
Application Number = 76302
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG
----
Product id = 25374
Application Number = 76302
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 150MG
----
Product id = 25376
Application Number = 76302
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode =
Rld = No
Strength = 200MG
----
Product id = 25378
Application Number = 76302
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = DISCN
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 005
Tecode =
Rld = No
Strength = 250MG
----
Product id = 25345
Application Number = 76309
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 001
Tecode = BX
Rld = No
Strength = 50MG
----
Product id = 25346
Application Number = 76309
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 002
Tecode = BX
Rld = No
Strength = 100MG
----
Product id = 25347
Application Number = 76309
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 003
Tecode = BX
Rld = No
Strength = 150MG
----
Product id = 25348
Application Number = 76309
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 004
Tecode = BX
Rld = No
Strength = 200MG
----
Product id = 25349
Application Number = 76309
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS INC
ProductNo = 005
Tecode = BX
Rld = No
Strength = 250MG
----
Product id = 25359
Application Number = 76409
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 50MG
----
Product id = 25360
Application Number = 76409
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 100MG
----
Product id = 25361
Application Number = 76409
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 003
Tecode = AB
Rld = No
Strength = 150MG
----
Product id = 25362
Application Number = 76409
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 004
Tecode = AB
Rld = No
Strength = 200MG
----
Product id = 25363
Application Number = 76409
Date of Application = 16,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = NEFAZODONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 005
Tecode = AB
Rld = No
Strength = 250MG
----