paroxetine-hydrochloride
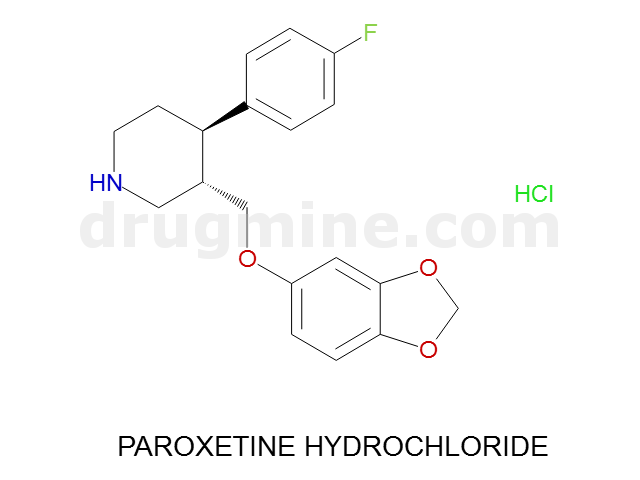
Name: PAROXETINE HYDROCHLORIDE
ID :
MW: 329
Number of atoms: 24
Molecular_Formula: C19H20FNO3
Alogp: 3.229
Indication class : .
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
paroxetine hydrochloride containing products summary
There are in total 65 different products containing the active ingredient paroxetine hydrochloride. From the 65 drug products, 18 have been discontinued.Product id = 25950
Application Number = 20031
Date of Application = 29,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = PAXIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTEX TECHNOLOGIES
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 25951
Application Number = 20031
Date of Application = 29,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = PAXIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTEX TECHNOLOGIES
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 25952
Application Number = 20031
Date of Application = 29,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = PAXIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTEX TECHNOLOGIES
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 25954
Application Number = 20031
Date of Application = 29,, Dec, 1992
RX/OTC/DISCN = DISCN
Tradename = PAXIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTEX TECHNOLOGIES
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 50MG BASE
----
Product id = 25953
Application Number = 20031
Date of Application = 29,, Dec, 1992
RX/OTC/DISCN = RX
Tradename = PAXIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTEX TECHNOLOGIES
ProductNo = 005
Tecode = AB
Rld = Yes
Strength = EQ 40MG BASE
----
Product id = 14782
Application Number = 20710
Date of Application = 25,, Jun, 1997
RX/OTC/DISCN = RX
Tradename = PAXIL
Route/format = ORAL / SUSPENSION
Application Type = N
Applicant Name = APOTEX TECHNOLOGIES
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 10MG BASE/5ML
----
Product id = 2790
Application Number = 20885
Date of Application = 9,, Oct, 1998
RX/OTC/DISCN = DISCN
Tradename = PAXIL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = APOTEX TECHNOLOGIES
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 2791
Application Number = 20885
Date of Application = 9,, Oct, 1998
RX/OTC/DISCN = DISCN
Tradename = PAXIL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = APOTEX TECHNOLOGIES
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 2792
Application Number = 20885
Date of Application = 9,, Oct, 1998
RX/OTC/DISCN = DISCN
Tradename = PAXIL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = APOTEX TECHNOLOGIES
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 30MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 2793
Application Number = 20885
Date of Application = 9,, Oct, 1998
RX/OTC/DISCN = DISCN
Tradename = PAXIL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = APOTEX TECHNOLOGIES
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 40MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 16477
Application Number = 20936
Date of Application = 16,, Feb, 1999
RX/OTC/DISCN = RX
Tradename = PAXIL CR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = APOTEX TECHNOLOGIES
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 12.5MG BASE
----
Product id = 16478
Application Number = 20936
Date of Application = 16,, Feb, 1999
RX/OTC/DISCN = RX
Tradename = PAXIL CR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = APOTEX TECHNOLOGIES
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 25MG BASE
----
Product id = 16479
Application Number = 20936
Date of Application = 6,, Dec, 2000
RX/OTC/DISCN = RX
Tradename = PAXIL CR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = APOTEX TECHNOLOGIES
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = EQ 37.5MG BASE
----
Product id = 25901
Application Number = 75356
Date of Application = 30,, Jul, 2003
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 25902
Application Number = 75356
Date of Application = 30,, Jul, 2003
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 25903
Application Number = 75356
Date of Application = 30,, Jul, 2003
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 25904
Application Number = 75356
Date of Application = 30,, Jul, 2003
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 25925
Application Number = 75566
Date of Application = 8,, Mar, 2004
RX/OTC/DISCN = DISCN
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 25926
Application Number = 75566
Date of Application = 8,, Mar, 2004
RX/OTC/DISCN = DISCN
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20MG BASE
----
Product id = 25927
Application Number = 75566
Date of Application = 8,, Mar, 2004
RX/OTC/DISCN = DISCN
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 30MG BASE
----
Product id = 25928
Application Number = 75566
Date of Application = 8,, Mar, 2004
RX/OTC/DISCN = DISCN
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 40MG BASE
----
Product id = 25913
Application Number = 75716
Date of Application = 8,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 25914
Application Number = 75716
Date of Application = 8,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 25915
Application Number = 75716
Date of Application = 8,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 25916
Application Number = 75716
Date of Application = 8,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 25933
Application Number = 76618
Date of Application = 15,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 25934
Application Number = 76618
Date of Application = 15,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 25935
Application Number = 76618
Date of Application = 15,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 25936
Application Number = 76618
Date of Application = 15,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 25917
Application Number = 76968
Date of Application = 21,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 25918
Application Number = 76968
Date of Application = 21,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 25919
Application Number = 76968
Date of Application = 21,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 25920
Application Number = 76968
Date of Application = 21,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 25937
Application Number = 77082
Date of Application = 29,, Jun, 2007
RX/OTC/DISCN = DISCN
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 25938
Application Number = 77082
Date of Application = 29,, Jun, 2007
RX/OTC/DISCN = DISCN
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20MG BASE
----
Product id = 25939
Application Number = 77082
Date of Application = 29,, Jun, 2007
RX/OTC/DISCN = DISCN
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 30MG BASE
----
Product id = 25940
Application Number = 77082
Date of Application = 29,, Jun, 2007
RX/OTC/DISCN = DISCN
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 40MG BASE
----
Product id = 14781
Application Number = 77395
Date of Application = 5,, Dec, 2006
RX/OTC/DISCN = DISCN
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / SUSPENSION
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE/5ML
----
Product id = 25941
Application Number = 77584
Date of Application = 7,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 25942
Application Number = 77584
Date of Application = 7,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 25943
Application Number = 77584
Date of Application = 7,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 25944
Application Number = 77584
Date of Application = 7,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 16474
Application Number = 77873
Date of Application = 29,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 12.5MG BASE
----
Product id = 16475
Application Number = 77873
Date of Application = 29,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 25MG BASE
----
Product id = 25921
Application Number = 78026
Date of Application = 29,, Jun, 2007
RX/OTC/DISCN = DISCN
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 25922
Application Number = 78026
Date of Application = 29,, Jun, 2007
RX/OTC/DISCN = DISCN
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20MG BASE
----
Product id = 25923
Application Number = 78026
Date of Application = 29,, Jun, 2007
RX/OTC/DISCN = DISCN
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 30MG BASE
----
Product id = 25924
Application Number = 78026
Date of Application = 29,, Jun, 2007
RX/OTC/DISCN = DISCN
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 40MG BASE
----
Product id = 25929
Application Number = 78194
Date of Application = 29,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 25930
Application Number = 78194
Date of Application = 29,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 25931
Application Number = 78194
Date of Application = 29,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 25932
Application Number = 78194
Date of Application = 29,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 25905
Application Number = 78406
Date of Application = 25,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 25906
Application Number = 78406
Date of Application = 25,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 25907
Application Number = 78406
Date of Application = 25,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 25908
Application Number = 78406
Date of Application = 25,, Jul, 2007
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 25909
Application Number = 78902
Date of Application = 13,, Mar, 2008
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 25910
Application Number = 78902
Date of Application = 13,, Mar, 2008
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 25911
Application Number = 78902
Date of Application = 13,, Mar, 2008
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 25912
Application Number = 78902
Date of Application = 13,, Mar, 2008
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 16476
Application Number = 91427
Date of Application = 14,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = PAROXETINE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 37.5MG BASE
----
Product id = 25897
Application Number = 203854
Date of Application = 31,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = PAROXETINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 25898
Application Number = 203854
Date of Application = 31,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = PAROXETINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 25899
Application Number = 203854
Date of Application = 31,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = PAROXETINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 30MG BASE
----
Product id = 25900
Application Number = 203854
Date of Application = 31,, Oct, 2014
RX/OTC/DISCN = RX
Tradename = PAROXETINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----