perindopril-erbumine
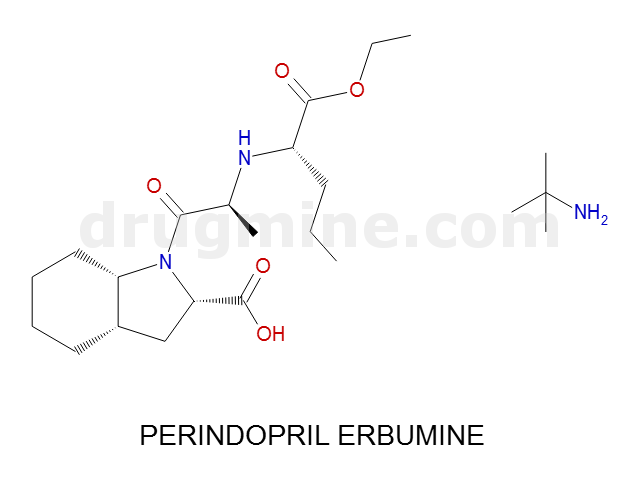
Name: PERINDOPRIL ERBUMINE
ID :
MW: 368
Number of atoms: 26
Molecular_Formula: C19H32N2O5
Alogp: -0.268
Indication class : Antihypertensive
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
perindopril erbumine containing products summary
There are in total 18 different products containing the active ingredient perindopril erbumine. From the 18 drug products, zero have been discontinued.Product id = 17205
Application Number = 20184
Date of Application = 30,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = ACEON
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SYMPLMED PHARMS LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 17206
Application Number = 20184
Date of Application = 30,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = ACEON
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SYMPLMED PHARMS LLC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 17207
Application Number = 20184
Date of Application = 30,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = ACEON
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SYMPLMED PHARMS LLC
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 8MG
----
Product id = 26053
Application Number = 78138
Date of Application = 10,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = PERINDOPRIL ERBUMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 26054
Application Number = 78138
Date of Application = 10,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = PERINDOPRIL ERBUMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 26055
Application Number = 78138
Date of Application = 10,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = PERINDOPRIL ERBUMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 8MG
----
Product id = 26062
Application Number = 78263
Date of Application = 27,, Jan, 2010
RX/OTC/DISCN = RX
Tradename = PERINDOPRIL ERBUMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 26063
Application Number = 78263
Date of Application = 27,, Jan, 2010
RX/OTC/DISCN = RX
Tradename = PERINDOPRIL ERBUMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 26064
Application Number = 78263
Date of Application = 27,, Jan, 2010
RX/OTC/DISCN = RX
Tradename = PERINDOPRIL ERBUMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 8MG
----
Product id = 26059
Application Number = 79070
Date of Application = 10,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = PERINDOPRIL ERBUMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 26060
Application Number = 79070
Date of Application = 10,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = PERINDOPRIL ERBUMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 26061
Application Number = 79070
Date of Application = 10,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = PERINDOPRIL ERBUMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 8MG
----
Product id = 26065
Application Number = 90072
Date of Application = 10,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = PERINDOPRIL ERBUMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 26066
Application Number = 90072
Date of Application = 10,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = PERINDOPRIL ERBUMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 26067
Application Number = 90072
Date of Application = 10,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = PERINDOPRIL ERBUMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ROXANE
ProductNo = 003
Tecode = AB
Rld = No
Strength = 8MG
----
Product id = 26056
Application Number = 90463
Date of Application = 30,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = PERINDOPRIL ERBUMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 26057
Application Number = 90463
Date of Application = 30,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = PERINDOPRIL ERBUMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 26058
Application Number = 90463
Date of Application = 30,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = PERINDOPRIL ERBUMINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 003
Tecode = AB
Rld = No
Strength = 8MG
----