perphenazine
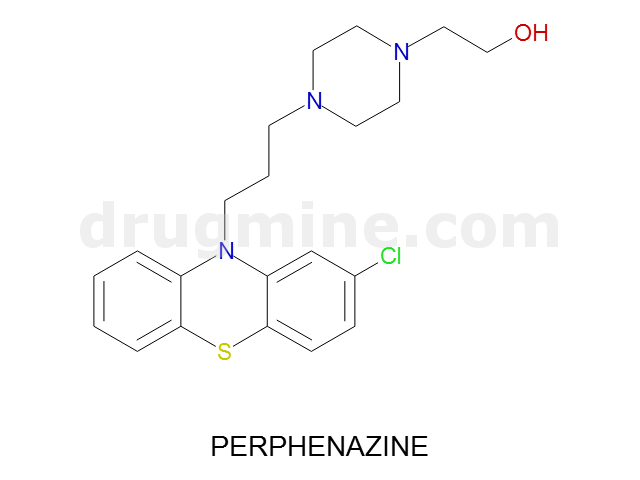

Name: PERPHENAZINE
ID :
MW: 404
Number of atoms: 27
Molecular_Formula: C21H26ClN3OS
Alogp: 4.157
Indication class : Antipsychotic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
perphenazine containing products summary
There are in total 69 different products containing the active ingredient perphenazine. From the 69 drug products, 56 have been discontinued.Product id = 29211
Application Number = 10775
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRILAFON
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 29212
Application Number = 10775
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRILAFON
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SCHERING
ProductNo = 002
Tecode =
Rld = No
Strength = 4MG
----
Product id = 29213
Application Number = 10775
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRILAFON
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SCHERING
ProductNo = 003
Tecode =
Rld = No
Strength = 8MG
----
Product id = 29214
Application Number = 10775
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRILAFON
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SCHERING
ProductNo = 004
Tecode =
Rld = No
Strength = 16MG
----
Product id = 11209
Application Number = 11213
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRILAFON
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = SCHERING
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG/ML
----
Product id = 15160
Application Number = 11294
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRILAFON
Route/format = ORAL / SYRUP
Application Type = N
Applicant Name = SCHERING
ProductNo = 002
Tecode =
Rld = No
Strength = 2MG/5ML
----
Product id = 16730
Application Number = 11361
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRILAFON
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SCHERING
ProductNo = 002
Tecode =
Rld = No
Strength = 8MG
----
Product id = 3999
Application Number = 11557
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRILAFON
Route/format = ORAL / CONCENTRATE
Application Type = N
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = 16MG/5ML
----
Product id = 21111
Application Number = 14713
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ETRAFON-A
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SCHERING
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG;4MG
----
Product id = 21110
Application Number = 14713
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ETRAFON 2-25
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SCHERING
ProductNo = 004
Tecode =
Rld = No
Strength = 25MG;2MG
----
Product id = 21112
Application Number = 14713
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ETRAFON-FORTE
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SCHERING
ProductNo = 006
Tecode =
Rld = No
Strength = 25MG;4MG
----
Product id = 21109
Application Number = 14713
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ETRAFON 2-10
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SCHERING
ProductNo = 007
Tecode =
Rld = No
Strength = 10MG;2MG
----
Product id = 29136
Application Number = 14715
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRIAVIL 2-25
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NEW RIVER
ProductNo = 002
Tecode =
Rld = No
Strength = 25MG;2MG
----
Product id = 29137
Application Number = 14715
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRIAVIL 4-10
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NEW RIVER
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG;4MG
----
Product id = 29135
Application Number = 14715
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRIAVIL 2-10
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NEW RIVER
ProductNo = 004
Tecode =
Rld = No
Strength = 10MG;2MG
----
Product id = 29138
Application Number = 14715
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRIAVIL 4-25
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NEW RIVER
ProductNo = 005
Tecode =
Rld = No
Strength = 25MG;4MG
----
Product id = 29139
Application Number = 14715
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TRIAVIL 4-50
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = NEW RIVER
ProductNo = 006
Tecode =
Rld = No
Strength = 50MG;4MG
----
Product id = 26084
Application Number = 40226
Date of Application = 31,, Dec, 1998
RX/OTC/DISCN = RX
Tradename = PERPHENAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 26085
Application Number = 40226
Date of Application = 31,, Dec, 1998
RX/OTC/DISCN = RX
Tradename = PERPHENAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 26086
Application Number = 40226
Date of Application = 31,, Dec, 1998
RX/OTC/DISCN = RX
Tradename = PERPHENAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 8MG
----
Product id = 26087
Application Number = 40226
Date of Application = 31,, Dec, 1998
RX/OTC/DISCN = RX
Tradename = PERPHENAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 16MG
----
Product id = 3958
Application Number = 40360
Date of Application = 25,, May, 2001
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE
Route/format = ORAL / CONCENTRATE
Application Type = A
Applicant Name = PHARM ASSOC
ProductNo = 001
Tecode =
Rld = No
Strength = 16MG/5ML
----
Product id = 26095
Application Number = 70297
Date of Application = 12,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;2MG
----
Product id = 26112
Application Number = 70373
Date of Application = 25,, Aug, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;2MG
----
Product id = 26118
Application Number = 70374
Date of Application = 25,, Aug, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;2MG
----
Product id = 26115
Application Number = 70375
Date of Application = 25,, Aug, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;4MG
----
Product id = 26121
Application Number = 70376
Date of Application = 25,, Aug, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;4MG
----
Product id = 26124
Application Number = 70377
Date of Application = 4,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;4MG
----
Product id = 26102
Application Number = 70565
Date of Application = 11,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;2MG
----
Product id = 26106
Application Number = 70574
Date of Application = 11,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;4MG
----
Product id = 26105
Application Number = 70595
Date of Application = 11,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;4MG
----
Product id = 26103
Application Number = 70620
Date of Application = 11,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;4MG
----
Product id = 26104
Application Number = 70621
Date of Application = 11,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;2MG
----
Product id = 26088
Application Number = 70935
Date of Application = 11,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;2MG
----
Product id = 26090
Application Number = 70936
Date of Application = 11,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;2MG
----
Product id = 26089
Application Number = 70937
Date of Application = 11,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;4MG
----
Product id = 26091
Application Number = 70938
Date of Application = 11,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;4MG
----
Product id = 26092
Application Number = 70939
Date of Application = 12,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;4MG
----
Product id = 26107
Application Number = 71062
Date of Application = 27,, Nov, 1987
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;2MG
----
Product id = 26109
Application Number = 71063
Date of Application = 27,, Nov, 1987
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;2MG
----
Product id = 26110
Application Number = 71064
Date of Application = 27,, Nov, 1987
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;4MG
----
Product id = 26093
Application Number = 71077
Date of Application = 12,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;2MG
----
Product id = 26094
Application Number = 71078
Date of Application = 12,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;4MG
----
Product id = 26096
Application Number = 71079
Date of Application = 12,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;4MG
----
Product id = 26101
Application Number = 71443
Date of Application = 10,, Nov, 1988
RX/OTC/DISCN = RX
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = Yes
Strength = 50MG;4MG
----
Product id = 26097
Application Number = 71443
Date of Application = 10,, Nov, 1988
RX/OTC/DISCN = RX
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG;2MG
----
Product id = 26098
Application Number = 71443
Date of Application = 10,, Nov, 1988
RX/OTC/DISCN = RX
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG;4MG
----
Product id = 26099
Application Number = 71443
Date of Application = 10,, Nov, 1988
RX/OTC/DISCN = RX
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode =
Rld = Yes
Strength = 25MG;2MG
----
Product id = 26100
Application Number = 71443
Date of Application = 10,, Nov, 1988
RX/OTC/DISCN = RX
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 005
Tecode =
Rld = Yes
Strength = 25MG;4MG
----
Product id = 26125
Application Number = 71558
Date of Application = 2,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;4MG
----
Product id = 26108
Application Number = 71862
Date of Application = 21,, Dec, 1987
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;4MG
----
Product id = 26111
Application Number = 71863
Date of Application = 21,, Dec, 1987
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;4MG
----
Product id = 26122
Application Number = 72134
Date of Application = 15,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;4MG
----
Product id = 26126
Application Number = 72135
Date of Application = 15,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG;4MG
----
Product id = 26113
Application Number = 72539
Date of Application = 15,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;2MG
----
Product id = 26116
Application Number = 72540
Date of Application = 15,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;4MG
----
Product id = 26119
Application Number = 72541
Date of Application = 15,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;2MG
----
Product id = 26114
Application Number = 73007
Date of Application = 17,, Oct, 1991
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;2MG
----
Product id = 26120
Application Number = 73008
Date of Application = 17,, Oct, 1991
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;2MG
----
Product id = 26117
Application Number = 73009
Date of Application = 17,, Oct, 1991
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 10MG;4MG
----
Product id = 26123
Application Number = 73010
Date of Application = 17,, Oct, 1991
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 25MG;4MG
----
Product id = 26076
Application Number = 89456
Date of Application = 10,, Sep, 1987
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 8MG
----
Product id = 26083
Application Number = 89457
Date of Application = 10,, Sep, 1987
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode =
Rld = No
Strength = 16MG
----
Product id = 26077
Application Number = 89683
Date of Application = 8,, Dec, 1988
RX/OTC/DISCN = RX
Tradename = PERPHENAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 26078
Application Number = 89684
Date of Application = 8,, Dec, 1988
RX/OTC/DISCN = RX
Tradename = PERPHENAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 26079
Application Number = 89685
Date of Application = 8,, Dec, 1988
RX/OTC/DISCN = RX
Tradename = PERPHENAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 8MG
----
Product id = 26080
Application Number = 89686
Date of Application = 8,, Dec, 1988
RX/OTC/DISCN = RX
Tradename = PERPHENAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 16MG
----
Product id = 26081
Application Number = 89707
Date of Application = 10,, Sep, 1987
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode =
Rld = No
Strength = 2MG
----
Product id = 26082
Application Number = 89708
Date of Application = 10,, Sep, 1987
RX/OTC/DISCN = DISCN
Tradename = PERPHENAZINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode =
Rld = No
Strength = 4MG
----