phenytoin-sodium
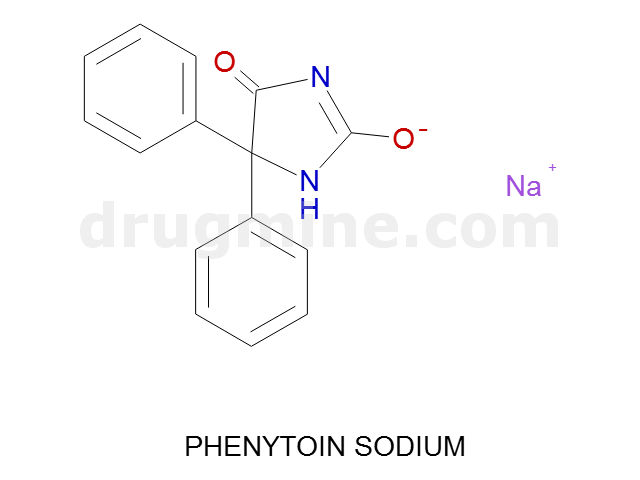
Name: PHENYTOIN SODIUM
ID :
MW: 251
Number of atoms: 19
Molecular_Formula: C15H11N2O2
Alogp: 2.084
Indication class : Anticonvulsant
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
phenytoin sodium containing products summary
There are in total 35 different products containing the active ingredient phenytoin sodium. From the 35 drug products, 19 have been discontinued.Product id = 7236
Application Number = 10151
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DILANTIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 1902
Application Number = 40298
Date of Application = 28,, Dec, 1998
RX/OTC/DISCN = RX
Tradename = EXTENDED PHENYTOIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG EXTENDED
----
Product id = 2890
Application Number = 40298
Date of Application = 6,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = PHENYTEK
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 200MG EXTENDED
----
Product id = 2891
Application Number = 40298
Date of Application = 6,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = PHENYTEK
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 300MG EXTENDED
----
Product id = 1900
Application Number = 40435
Date of Application = 20,, Jun, 2003
RX/OTC/DISCN = DISCN
Tradename = EXTENDED PHENYTOIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG EXTENDED
----
Product id = 10123
Application Number = 40573
Date of Application = 13,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = PHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = X-GEN PHARMS
ProductNo = 001
Tecode = AP
Rld = No
Strength = 50MG/ML
----
Product id = 1905
Application Number = 40621
Date of Application = 11,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = EXTENDED PHENYTOIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SUN PHARM INDS (IN)
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG EXTENDED
----
Product id = 1906
Application Number = 40684
Date of Application = 5,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = EXTENDED PHENYTOIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG EXTENDED
----
Product id = 1903
Application Number = 40731
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = EXTENDED PHENYTOIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SUN PHARM INDS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 200MG EXTENDED
----
Product id = 1904
Application Number = 40731
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = EXTENDED PHENYTOIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SUN PHARM INDS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 300MG EXTENDED
----
Product id = 1908
Application Number = 40732
Date of Application = 30,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = EXTENDED PHENYTOIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WOCKHARDT USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG EXTENDED
----
Product id = 1907
Application Number = 40759
Date of Application = 18,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = EXTENDED PHENYTOIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 30MG EXTENDED
----
Product id = 1899
Application Number = 40765
Date of Application = 12,, Nov, 2008
RX/OTC/DISCN = RX
Tradename = EXTENDED PHENYTOIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = AMNEAL PHARMS NY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 100MG EXTENDED
----
Product id = 10115
Application Number = 40781
Date of Application = 4,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = PHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUITPOLD
ProductNo = 001
Tecode = AP
Rld = No
Strength = 50MG/ML
----
Product id = 3042
Application Number = 80259
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROMPT PHENYTOIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG PROMPT
----
Product id = 1669
Application Number = 80857
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DIPHENYLAN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode =
Rld = No
Strength = 30MG PROMPT
----
Product id = 1670
Application Number = 80857
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DIPHENYLAN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LANNETT
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG PROMPT
----
Product id = 3043
Application Number = 80905
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROMPT PHENYTOIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG PROMPT
----
Product id = 10111
Application Number = 84307
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = PHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = EUROHLTH INTL SARL
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = 50MG/ML
----
Product id = 1606
Application Number = 84349
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DILANTIN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 30MG EXTENDED
----
Product id = 1607
Application Number = 84349
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DILANTIN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 100MG EXTENDED
----
Product id = 10122
Application Number = 85434
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 2893
Application Number = 85435
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PHENYTOIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PHARMERAL
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG PROMPT
----
Product id = 2894
Application Number = 85894
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PHENYTOIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG PROMPT
----
Product id = 10118
Application Number = 88519
Date of Application = 19,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = PHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SMITH AND NEPHEW
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 10120
Application Number = 88520
Date of Application = 17,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = PHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SOLOPAK
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 10119
Application Number = 88521
Date of Application = 18,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = PHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SMITH AND NEPHEW
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 2892
Application Number = 88711
Date of Application = 21,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = PHENYTEX
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG EXTENDED
----
Product id = 10112
Application Number = 89003
Date of Application = 31,, May, 1985
RX/OTC/DISCN = DISCN
Tradename = PHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 1901
Application Number = 89441
Date of Application = 18,, Dec, 1986
RX/OTC/DISCN = DISCN
Tradename = EXTENDED PHENYTOIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG EXTENDED
----
Product id = 10116
Application Number = 89501
Date of Application = 13,, Oct, 1987
RX/OTC/DISCN = DISCN
Tradename = PHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MARSAM PHARMS LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 10113
Application Number = 89521
Date of Application = 17,, Mar, 1987
RX/OTC/DISCN = RX
Tradename = PHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = 50MG/ML
----
Product id = 10114
Application Number = 89744
Date of Application = 18,, Dec, 1987
RX/OTC/DISCN = DISCN
Tradename = PHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 10117
Application Number = 89779
Date of Application = 27,, Nov, 1992
RX/OTC/DISCN = DISCN
Tradename = PHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MARSAM PHARMS LLC
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/ML
----
Product id = 10121
Application Number = 89900
Date of Application = 30,, Mar, 1990
RX/OTC/DISCN = DISCN
Tradename = PHENYTOIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 50MG/ML
----