procainamide-hydrochloride
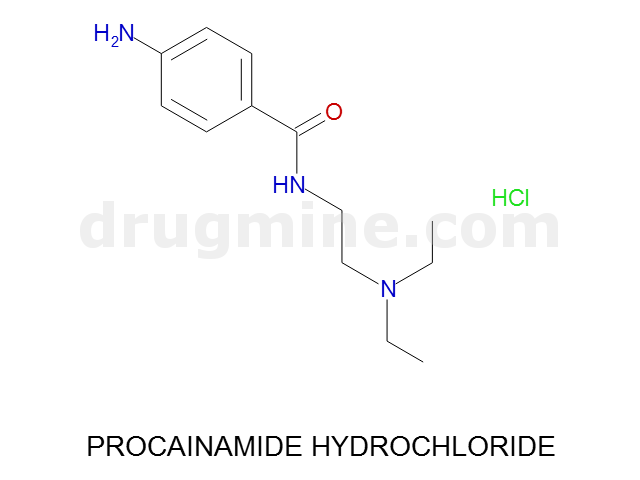

Name: PROCAINAMIDE HYDROCHLORIDE
ID :
MW: 235
Number of atoms: 17
Molecular_Formula: C13H21N3O
Alogp: 1.127
Indication class : Cardiac Depressant (anti-arrhythmic)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
procainamide hydrochloride containing products summary
There are in total 80 different products containing the active ingredient procainamide hydrochloride. From the 80 drug products, 78 have been discontinued.Product id = 3044
Application Number = 7335
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PRONESTYL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 10549
Application Number = 7335
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PRONESTYL
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = 100MG/ML
----
Product id = 3046
Application Number = 7335
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PRONESTYL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = APOTHECON
ProductNo = 003
Tecode =
Rld = No
Strength = 500MG
----
Product id = 3045
Application Number = 7335
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PRONESTYL
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = APOTHECON
ProductNo = 004
Tecode =
Rld = No
Strength = 375MG
----
Product id = 10550
Application Number = 7335
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PRONESTYL
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = APOTHECON
ProductNo = 005
Tecode =
Rld = No
Strength = 500MG/ML
----
Product id = 26822
Application Number = 17371
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PRONESTYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 26823
Application Number = 17371
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PRONESTYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = 375MG
----
Product id = 26824
Application Number = 17371
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PRONESTYL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = APOTHECON
ProductNo = 003
Tecode =
Rld = No
Strength = 500MG
----
Product id = 16545
Application Number = 20545
Date of Application = 31,, Jan, 1996
RX/OTC/DISCN = DISCN
Tradename = PROCANBID
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = KING PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 16544
Application Number = 20545
Date of Application = 31,, Jan, 1996
RX/OTC/DISCN = DISCN
Tradename = PROCANBID
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = KING PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 1GM
----
Product id = 16523
Application Number = 40111
Date of Application = 13,, Dec, 1996
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 1GM
----
Product id = 3014
Application Number = 83287
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 3025
Application Number = 83553
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAPAN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PANRAY
ProductNo = 002
Tecode =
Rld = No
Strength = 250MG
----
Product id = 3002
Application Number = 83693
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 3015
Application Number = 83795
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 3019
Application Number = 84280
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 3020
Application Number = 84357
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 3017
Application Number = 84403
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 375MG
----
Product id = 3000
Application Number = 84595
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 375MG
----
Product id = 2999
Application Number = 84604
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 3001
Application Number = 84606
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 3003
Application Number = 84696
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LANNETT
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 3024
Application Number = 85079
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 3016
Application Number = 85167
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 3022
Application Number = 85804
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 16542
Application Number = 86065
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAN SR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = PARKEDALE
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 16540
Application Number = 86468
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAN SR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 3004
Application Number = 86942
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 3006
Application Number = 86943
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 3005
Application Number = 86952
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = LEDERLE
ProductNo = 001
Tecode =
Rld = No
Strength = 375MG
----
Product id = 3018
Application Number = 87020
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 375MG
----
Product id = 3021
Application Number = 87021
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 10471
Application Number = 87079
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/ML
----
Product id = 10472
Application Number = 87080
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG/ML
----
Product id = 16549
Application Number = 87361
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PRONESTYL-SR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 3023
Application Number = 87502
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PROCAN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PARKE DAVIS
ProductNo = 001
Tecode =
Rld = No
Strength = 375MG
----
Product id = 16543
Application Number = 87510
Date of Application = 1,, Apr, 1982
RX/OTC/DISCN = DISCN
Tradename = PROCAN SR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = PARKEDALE
ProductNo = 001
Tecode =
Rld = No
Strength = 750MG
----
Product id = 2996
Application Number = 87542
Date of Application = 8,, Jan, 1982
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ASCOT
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 2998
Application Number = 87543
Date of Application = 8,, Jan, 1982
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ASCOT
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 3012
Application Number = 87643
Date of Application = 1,, Jun, 1982
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = VANGARD
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 2997
Application Number = 87697
Date of Application = 1,, Mar, 1983
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ASCOT
ProductNo = 001
Tecode =
Rld = No
Strength = 375MG
----
Product id = 3013
Application Number = 87875
Date of Application = 1,, Jun, 1982
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = VANGARD
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 16541
Application Number = 88489
Date of Application = 16,, Jan, 1985
RX/OTC/DISCN = DISCN
Tradename = PROCAN SR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = PARKEDALE
ProductNo = 001
Tecode =
Rld = No
Strength = 1GM
----
Product id = 10466
Application Number = 88530
Date of Application = 4,, Mar, 1985
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SMITH AND NEPHEW
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/ML
----
Product id = 10467
Application Number = 88531
Date of Application = 4,, Mar, 1985
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SMITH AND NEPHEW
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG/ML
----
Product id = 10468
Application Number = 88532
Date of Application = 4,, Mar, 1985
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SOLOPAK
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG/ML
----
Product id = 16534
Application Number = 88533
Date of Application = 3,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 16536
Application Number = 88534
Date of Application = 3,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 16538
Application Number = 88535
Date of Application = 3,, Nov, 1984
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 750MG
----
Product id = 10462
Application Number = 88636
Date of Application = 31,, Jul, 1984
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = INTL MEDICATION
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/ML
----
Product id = 10463
Application Number = 88637
Date of Application = 31,, Jul, 1984
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = INTL MEDICATION
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG/ML
----
Product id = 10464
Application Number = 88824
Date of Application = 20,, Nov, 1985
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = PHARMAFAIR
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/ML
----
Product id = 10465
Application Number = 88830
Date of Application = 20,, Nov, 1985
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = PHARMAFAIR
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG/ML
----
Product id = 16524
Application Number = 88958
Date of Application = 2,, Dec, 1985
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 16525
Application Number = 88959
Date of Application = 2,, Dec, 1985
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 16526
Application Number = 88974
Date of Application = 22,, Jul, 1985
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 3007
Application Number = 88989
Date of Application = 26,, Apr, 1985
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 3008
Application Number = 88990
Date of Application = 26,, Apr, 1985
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 16535
Application Number = 89026
Date of Application = 22,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 16537
Application Number = 89027
Date of Application = 22,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 10457
Application Number = 89029
Date of Application = 17,, Apr, 1986
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/ML
----
Product id = 10458
Application Number = 89030
Date of Application = 17,, Apr, 1986
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG/ML
----
Product id = 16539
Application Number = 89042
Date of Application = 22,, Oct, 1985
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 750MG
----
Product id = 10459
Application Number = 89069
Date of Application = 12,, Feb, 1986
RX/OTC/DISCN = RX
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 100MG/ML
----
Product id = 10460
Application Number = 89070
Date of Application = 12,, Feb, 1986
RX/OTC/DISCN = RX
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = Yes
Strength = 500MG/ML
----
Product id = 3009
Application Number = 89219
Date of Application = 1,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 3010
Application Number = 89220
Date of Application = 1,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 375MG
----
Product id = 3011
Application Number = 89221
Date of Application = 1,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 16530
Application Number = 89284
Date of Application = 23,, Jun, 1986
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 16529
Application Number = 89369
Date of Application = 14,, Aug, 1987
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 16531
Application Number = 89370
Date of Application = 9,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----
Product id = 16532
Application Number = 89371
Date of Application = 14,, Aug, 1987
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 750MG
----
Product id = 10455
Application Number = 89415
Date of Application = 17,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/ML
----
Product id = 10456
Application Number = 89416
Date of Application = 17,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG/ML
----
Product id = 16527
Application Number = 89438
Date of Application = 23,, Mar, 1987
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 750MG
----
Product id = 16533
Application Number = 89520
Date of Application = 15,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 1GM
----
Product id = 10469
Application Number = 89528
Date of Application = 3,, May, 1988
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 100MG/ML
----
Product id = 10470
Application Number = 89529
Date of Application = 3,, May, 1988
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG/ML
----
Product id = 10461
Application Number = 89537
Date of Application = 25,, Aug, 1987
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG/ML
----
Product id = 16528
Application Number = 89840
Date of Application = 6,, Mar, 1989
RX/OTC/DISCN = DISCN
Tradename = PROCAINAMIDE HYDROCHLORIDE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = INWOOD LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 500MG
----