quinapril-hydrochloride
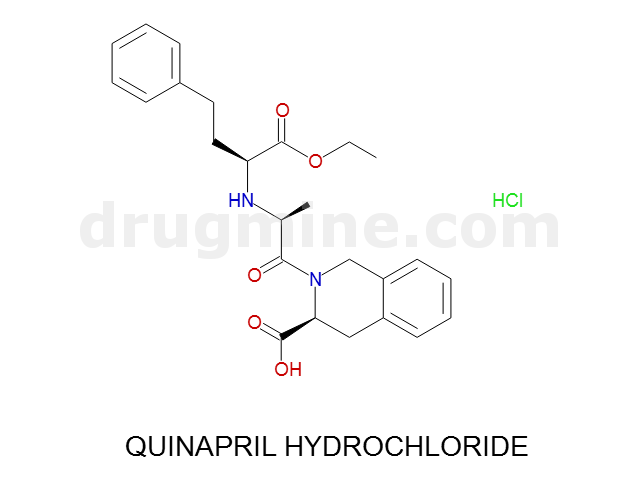


Name: QUINAPRIL HYDROCHLORIDE
ID :
MW: 439
Number of atoms: 32
Molecular_Formula: C25H30N2O5
Alogp: 0.656
Indication class : Enzyme Inhibitor (angiotensin-converting); Antihypertensive
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
quinapril hydrochloride containing products summary
There are in total 73 different products containing the active ingredient quinapril hydrochloride. From the 73 drug products, 16 have been discontinued.Product id = 17198
Application Number = 19885
Date of Application = 19,, Nov, 1991
RX/OTC/DISCN = RX
Tradename = ACCUPRIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 17199
Application Number = 19885
Date of Application = 19,, Nov, 1991
RX/OTC/DISCN = RX
Tradename = ACCUPRIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 17200
Application Number = 19885
Date of Application = 19,, Nov, 1991
RX/OTC/DISCN = RX
Tradename = ACCUPRIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 17201
Application Number = 19885
Date of Application = 19,, Nov, 1991
RX/OTC/DISCN = RX
Tradename = ACCUPRIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER PHARMS
ProductNo = 004
Tecode = AB
Rld = Yes
Strength = EQ 40MG BASE
----
Product id = 17202
Application Number = 20125
Date of Application = 28,, Dec, 1999
RX/OTC/DISCN = RX
Tradename = ACCURETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 10MG BASE
----
Product id = 17203
Application Number = 20125
Date of Application = 28,, Dec, 1999
RX/OTC/DISCN = RX
Tradename = ACCURETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 20MG BASE
----
Product id = 17204
Application Number = 20125
Date of Application = 28,, Dec, 1999
RX/OTC/DISCN = RX
Tradename = ACCURETIC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER PHARMS
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 25MG;EQ 20MG BASE
----
Product id = 27221
Application Number = 75504
Date of Application = 24,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27222
Application Number = 75504
Date of Application = 24,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27223
Application Number = 75504
Date of Application = 24,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27224
Application Number = 75504
Date of Application = 24,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 27201
Application Number = 76036
Date of Application = 28,, Jan, 2005
RX/OTC/DISCN = DISCN
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27203
Application Number = 76036
Date of Application = 28,, Jan, 2005
RX/OTC/DISCN = DISCN
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27205
Application Number = 76036
Date of Application = 28,, Jan, 2005
RX/OTC/DISCN = DISCN
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27207
Application Number = 76036
Date of Application = 28,, Jan, 2005
RX/OTC/DISCN = DISCN
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 40MG BASE
----
Product id = 27181
Application Number = 76049
Date of Application = 14,, Jan, 2005
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27182
Application Number = 76049
Date of Application = 14,, Jan, 2005
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27183
Application Number = 76049
Date of Application = 14,, Jan, 2005
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27184
Application Number = 76049
Date of Application = 14,, Jan, 2005
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 27185
Application Number = 76240
Date of Application = 26,, Jan, 2006
RX/OTC/DISCN = DISCN
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27186
Application Number = 76240
Date of Application = 26,, Jan, 2006
RX/OTC/DISCN = DISCN
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27187
Application Number = 76240
Date of Application = 26,, Jan, 2006
RX/OTC/DISCN = DISCN
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27188
Application Number = 76240
Date of Application = 26,, Jan, 2006
RX/OTC/DISCN = DISCN
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 40MG BASE
----
Product id = 27240
Application Number = 76374
Date of Application = 31,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = QUINARETIC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GAVIS PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 10MG BASE
----
Product id = 27241
Application Number = 76374
Date of Application = 31,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = QUINARETIC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GAVIS PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 20MG BASE
----
Product id = 27242
Application Number = 76374
Date of Application = 31,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = QUINARETIC
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GAVIS PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;EQ 20MG BASE
----
Product id = 27177
Application Number = 76459
Date of Application = 22,, Dec, 2004
RX/OTC/DISCN = DISCN
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27178
Application Number = 76459
Date of Application = 22,, Dec, 2004
RX/OTC/DISCN = DISCN
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27179
Application Number = 76459
Date of Application = 22,, Dec, 2004
RX/OTC/DISCN = DISCN
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27180
Application Number = 76459
Date of Application = 22,, Dec, 2004
RX/OTC/DISCN = DISCN
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 40MG BASE
----
Product id = 27209
Application Number = 76607
Date of Application = 15,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27210
Application Number = 76607
Date of Application = 15,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27211
Application Number = 76607
Date of Application = 15,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27212
Application Number = 76607
Date of Application = 15,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 27202
Application Number = 76694
Date of Application = 23,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27204
Application Number = 76694
Date of Application = 23,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27206
Application Number = 76694
Date of Application = 23,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27208
Application Number = 76694
Date of Application = 23,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 27213
Application Number = 76803
Date of Application = 2,, Mar, 2005
RX/OTC/DISCN = DISCN
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27214
Application Number = 76803
Date of Application = 2,, Mar, 2005
RX/OTC/DISCN = DISCN
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27215
Application Number = 76803
Date of Application = 2,, Mar, 2005
RX/OTC/DISCN = DISCN
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27216
Application Number = 76803
Date of Application = 2,, Mar, 2005
RX/OTC/DISCN = DISCN
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 40MG BASE
----
Product id = 27234
Application Number = 77093
Date of Application = 28,, Mar, 2005
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 10MG BASE
----
Product id = 27235
Application Number = 77093
Date of Application = 28,, Mar, 2005
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 20MG BASE
----
Product id = 27236
Application Number = 77093
Date of Application = 28,, Mar, 2005
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;EQ 20MG BASE
----
Product id = 27197
Application Number = 77690
Date of Application = 20,, Jun, 2006
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27198
Application Number = 77690
Date of Application = 20,, Jun, 2006
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27199
Application Number = 77690
Date of Application = 20,, Jun, 2006
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27200
Application Number = 77690
Date of Application = 20,, Jun, 2006
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 27237
Application Number = 78211
Date of Application = 4,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 10MG BASE
----
Product id = 27238
Application Number = 78211
Date of Application = 4,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 20MG BASE
----
Product id = 27239
Application Number = 78211
Date of Application = 4,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;EQ 20MG BASE
----
Product id = 27228
Application Number = 78450
Date of Application = 24,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 10MG BASE
----
Product id = 27229
Application Number = 78450
Date of Application = 24,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 20MG BASE
----
Product id = 27230
Application Number = 78450
Date of Application = 24,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;EQ 20MG BASE
----
Product id = 27193
Application Number = 78457
Date of Application = 24,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27194
Application Number = 78457
Date of Application = 24,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27195
Application Number = 78457
Date of Application = 24,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27196
Application Number = 78457
Date of Application = 24,, Aug, 2007
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 27217
Application Number = 90800
Date of Application = 18,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27218
Application Number = 90800
Date of Application = 18,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27219
Application Number = 90800
Date of Application = 18,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27220
Application Number = 90800
Date of Application = 18,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 27225
Application Number = 91524
Date of Application = 12,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX CORP
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 10MG BASE
----
Product id = 27226
Application Number = 91524
Date of Application = 12,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX CORP
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;EQ 20MG BASE
----
Product id = 27227
Application Number = 91524
Date of Application = 12,, Mar, 2013
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX CORP
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;EQ 20MG BASE
----
Product id = 27231
Application Number = 201356
Date of Application = 20,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;10MG
----
Product id = 27232
Application Number = 201356
Date of Application = 20,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;20MG
----
Product id = 27233
Application Number = 201356
Date of Application = 20,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = INVAGEN PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;20MG
----
Product id = 27189
Application Number = 202725
Date of Application = 29,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE
----
Product id = 27190
Application Number = 202725
Date of Application = 29,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 27191
Application Number = 202725
Date of Application = 29,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27192
Application Number = 202725
Date of Application = 29,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = QUINAPRIL HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----