risperidone
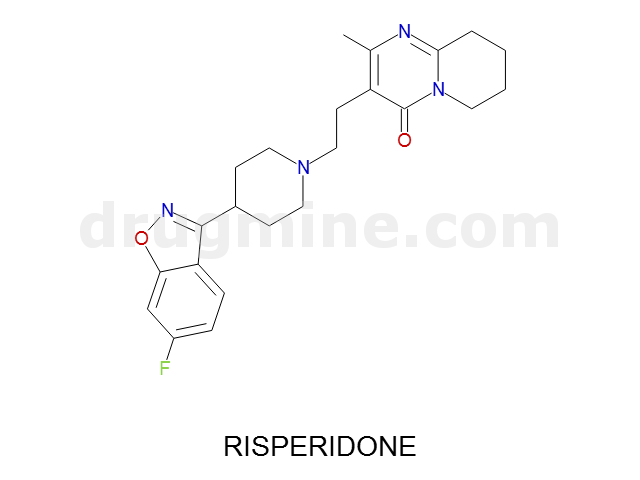

Name: RISPERIDONE
ID :
MW: 410
Number of atoms: 30
Molecular_Formula: C23H27FN4O2
Alogp: 3.317
Indication class : Neuroleptic
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
risperidone containing products summary
There are in total 200 different products containing the active ingredient risperidone. From the 200 drug products, 31 have been discontinued.Product id = 27555
Application Number = 20272
Date of Application = 29,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = RISPERDAL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 1MG
----
Product id = 27556
Application Number = 20272
Date of Application = 29,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = RISPERDAL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27557
Application Number = 20272
Date of Application = 29,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = RISPERDAL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 27558
Application Number = 20272
Date of Application = 29,, Dec, 1993
RX/OTC/DISCN = RX
Tradename = RISPERDAL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 27559
Application Number = 20272
Date of Application = 29,, Dec, 1993
RX/OTC/DISCN = DISCN
Tradename = RISPERDAL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 005
Tecode =
Rld = No
Strength = 5MG
----
Product id = 27554
Application Number = 20272
Date of Application = 27,, Jan, 1999
RX/OTC/DISCN = RX
Tradename = RISPERDAL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 007
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 27553
Application Number = 20272
Date of Application = 10,, May, 1999
RX/OTC/DISCN = RX
Tradename = RISPERDAL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 008
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 14021
Application Number = 20588
Date of Application = 10,, Jun, 1996
RX/OTC/DISCN = RX
Tradename = RISPERDAL
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = 1MG/ML
----
Product id = 11520
Application Number = 21346
Date of Application = 29,, Oct, 2003
RX/OTC/DISCN = RX
Tradename = RISPERDAL CONSTA
Route/format = INTRAMUSCULAR / INJECTABLE
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 25MG/VIAL
----
Product id = 11521
Application Number = 21346
Date of Application = 29,, Oct, 2003
RX/OTC/DISCN = RX
Tradename = RISPERDAL CONSTA
Route/format = INTRAMUSCULAR / INJECTABLE
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 37.5MG/VIAL
----
Product id = 11522
Application Number = 21346
Date of Application = 29,, Oct, 2003
RX/OTC/DISCN = RX
Tradename = RISPERDAL CONSTA
Route/format = INTRAMUSCULAR / INJECTABLE
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 50MG/VIAL
----
Product id = 11519
Application Number = 21346
Date of Application = 12,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = RISPERDAL CONSTA
Route/format = INTRAMUSCULAR / INJECTABLE
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = 12.5MG/VIAL
----
Product id = 17035
Application Number = 21444
Date of Application = 2,, Apr, 2003
RX/OTC/DISCN = RX
Tradename = RISPERDAL
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 17036
Application Number = 21444
Date of Application = 2,, Apr, 2003
RX/OTC/DISCN = RX
Tradename = RISPERDAL
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 1MG
----
Product id = 17037
Application Number = 21444
Date of Application = 2,, Apr, 2003
RX/OTC/DISCN = RX
Tradename = RISPERDAL
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 17038
Application Number = 21444
Date of Application = 23,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = RISPERDAL
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 17039
Application Number = 21444
Date of Application = 23,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = RISPERDAL
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = JANSSEN PHARMS
ProductNo = 005
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 27644
Application Number = 76228
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 27645
Application Number = 76228
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 27646
Application Number = 76228
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 27647
Application Number = 76228
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 004
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27648
Application Number = 76228
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 005
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 27649
Application Number = 76228
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 006
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 27596
Application Number = 76288
Date of Application = 15,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 27597
Application Number = 76288
Date of Application = 15,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 27598
Application Number = 76288
Date of Application = 15,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 27599
Application Number = 76288
Date of Application = 15,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27600
Application Number = 76288
Date of Application = 15,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 005
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 27601
Application Number = 76288
Date of Application = 15,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 006
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 14023
Application Number = 76440
Date of Application = 30,, Jan, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1MG/ML
----
Product id = 14027
Application Number = 76797
Date of Application = 28,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = PRECISION DOSE
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1MG/ML
----
Product id = 27590
Application Number = 76879
Date of Application = 24,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 27591
Application Number = 76879
Date of Application = 24,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 27592
Application Number = 76879
Date of Application = 24,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 27593
Application Number = 76879
Date of Application = 24,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27594
Application Number = 76879
Date of Application = 24,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 005
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 27595
Application Number = 76879
Date of Application = 24,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 006
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 14028
Application Number = 76904
Date of Application = 29,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1MG/ML
----
Product id = 17080
Application Number = 76908
Date of Application = 12,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 17081
Application Number = 76908
Date of Application = 12,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 17082
Application Number = 76908
Date of Application = 12,, Mar, 2012
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 17040
Application Number = 76996
Date of Application = 19,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 17041
Application Number = 76996
Date of Application = 19,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 17042
Application Number = 76996
Date of Application = 19,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 17043
Application Number = 76996
Date of Application = 19,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 17044
Application Number = 76996
Date of Application = 19,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 17045
Application Number = 77328
Date of Application = 24,, Feb, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 17046
Application Number = 77328
Date of Application = 5,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 17047
Application Number = 77328
Date of Application = 24,, Feb, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27608
Application Number = 77493
Date of Application = 29,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 27609
Application Number = 77493
Date of Application = 29,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 27610
Application Number = 77493
Date of Application = 29,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 27611
Application Number = 77493
Date of Application = 29,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27612
Application Number = 77493
Date of Application = 29,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 27613
Application Number = 77493
Date of Application = 29,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PRINSTON INC
ProductNo = 006
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 17059
Application Number = 77494
Date of Application = 30,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 17060
Application Number = 77494
Date of Application = 30,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 17061
Application Number = 77494
Date of Application = 26,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 17062
Application Number = 77494
Date of Application = 30,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 004
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 17063
Application Number = 77494
Date of Application = 30,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 005
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 17064
Application Number = 77494
Date of Application = 30,, Apr, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 006
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 17065
Application Number = 77542
Date of Application = 6,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 17066
Application Number = 77542
Date of Application = 6,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 17067
Application Number = 77542
Date of Application = 6,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = RANBAXY
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27584
Application Number = 77543
Date of Application = 18,, May, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 27585
Application Number = 77543
Date of Application = 18,, May, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 27586
Application Number = 77543
Date of Application = 18,, May, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 27587
Application Number = 77543
Date of Application = 18,, May, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA
ProductNo = 004
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27588
Application Number = 77543
Date of Application = 18,, May, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA
ProductNo = 005
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 27589
Application Number = 77543
Date of Application = 18,, May, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CIPLA
ProductNo = 006
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 14024
Application Number = 77719
Date of Application = 29,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1MG/ML
----
Product id = 27602
Application Number = 77769
Date of Application = 16,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 27603
Application Number = 77769
Date of Application = 16,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 27604
Application Number = 77769
Date of Application = 16,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 27605
Application Number = 77769
Date of Application = 16,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 004
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27606
Application Number = 77769
Date of Application = 16,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 005
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 27607
Application Number = 77769
Date of Application = 16,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 006
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 27620
Application Number = 77784
Date of Application = 8,, Jun, 2010
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RATIOPHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27621
Application Number = 77784
Date of Application = 8,, Jun, 2010
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RATIOPHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 27622
Application Number = 77784
Date of Application = 8,, Jun, 2010
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RATIOPHARM
ProductNo = 003
Tecode =
Rld = No
Strength = 1MG
----
Product id = 27623
Application Number = 77784
Date of Application = 8,, Jun, 2010
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RATIOPHARM
ProductNo = 004
Tecode =
Rld = No
Strength = 2MG
----
Product id = 27624
Application Number = 77784
Date of Application = 8,, Jun, 2010
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RATIOPHARM
ProductNo = 005
Tecode =
Rld = No
Strength = 3MG
----
Product id = 27625
Application Number = 77784
Date of Application = 8,, Jun, 2010
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RATIOPHARM
ProductNo = 006
Tecode =
Rld = No
Strength = 4MG
----
Product id = 27662
Application Number = 77860
Date of Application = 5,, Dec, 2008
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27663
Application Number = 77860
Date of Application = 5,, Dec, 2008
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 27664
Application Number = 77860
Date of Application = 5,, Dec, 2008
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 1MG
----
Product id = 27665
Application Number = 77860
Date of Application = 5,, Dec, 2008
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 004
Tecode =
Rld = No
Strength = 2MG
----
Product id = 27666
Application Number = 77860
Date of Application = 5,, Dec, 2008
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 005
Tecode =
Rld = No
Strength = 3MG
----
Product id = 27667
Application Number = 77860
Date of Application = 5,, Dec, 2008
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 006
Tecode =
Rld = No
Strength = 4MG
----
Product id = 27566
Application Number = 77953
Date of Application = 15,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 27567
Application Number = 77953
Date of Application = 15,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 27568
Application Number = 77953
Date of Application = 15,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 27569
Application Number = 77953
Date of Application = 15,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27570
Application Number = 77953
Date of Application = 15,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 27571
Application Number = 77953
Date of Application = 15,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 006
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 27632
Application Number = 78036
Date of Application = 10,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 27633
Application Number = 78036
Date of Application = 10,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 27634
Application Number = 78036
Date of Application = 10,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 27635
Application Number = 78036
Date of Application = 10,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27636
Application Number = 78036
Date of Application = 10,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 27637
Application Number = 78036
Date of Application = 10,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 006
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 27680
Application Number = 78040
Date of Application = 16,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 27681
Application Number = 78040
Date of Application = 16,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 27682
Application Number = 78040
Date of Application = 16,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 27683
Application Number = 78040
Date of Application = 16,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27684
Application Number = 78040
Date of Application = 16,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 27685
Application Number = 78040
Date of Application = 16,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 006
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 27614
Application Number = 78071
Date of Application = 17,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 27615
Application Number = 78071
Date of Application = 17,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 27616
Application Number = 78071
Date of Application = 17,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 27617
Application Number = 78071
Date of Application = 17,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27618
Application Number = 78071
Date of Application = 17,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 005
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 27619
Application Number = 78071
Date of Application = 17,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 006
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 17070
Application Number = 78116
Date of Application = 22,, Dec, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 17071
Application Number = 78116
Date of Application = 22,, Dec, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 17072
Application Number = 78116
Date of Application = 22,, Dec, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 17073
Application Number = 78116
Date of Application = 22,, Dec, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 17074
Application Number = 78116
Date of Application = 22,, Dec, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SANDOZ
ProductNo = 005
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 27638
Application Number = 78187
Date of Application = 22,, Oct, 2009
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SYNTHON PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27639
Application Number = 78187
Date of Application = 22,, Oct, 2009
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SYNTHON PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 27640
Application Number = 78187
Date of Application = 22,, Oct, 2009
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SYNTHON PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 1MG
----
Product id = 27641
Application Number = 78187
Date of Application = 22,, Oct, 2009
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SYNTHON PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = 2MG
----
Product id = 27642
Application Number = 78187
Date of Application = 22,, Oct, 2009
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SYNTHON PHARMS
ProductNo = 005
Tecode =
Rld = No
Strength = 3MG
----
Product id = 27643
Application Number = 78187
Date of Application = 22,, Oct, 2009
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SYNTHON PHARMS
ProductNo = 006
Tecode =
Rld = No
Strength = 4MG
----
Product id = 27572
Application Number = 78269
Date of Application = 8,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 27573
Application Number = 78269
Date of Application = 8,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 27574
Application Number = 78269
Date of Application = 8,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 27575
Application Number = 78269
Date of Application = 8,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 004
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27576
Application Number = 78269
Date of Application = 8,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 005
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 27577
Application Number = 78269
Date of Application = 8,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 006
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 14025
Application Number = 78452
Date of Application = 4,, Sep, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1MG/ML
----
Product id = 17075
Application Number = 78464
Date of Application = 8,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SUN PHARM INDS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 17076
Application Number = 78464
Date of Application = 8,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SUN PHARM INDS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 17077
Application Number = 78464
Date of Application = 8,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SUN PHARM INDS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 17078
Application Number = 78464
Date of Application = 8,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SUN PHARM INDS LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 17079
Application Number = 78464
Date of Application = 8,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = SUN PHARM INDS LTD
ProductNo = 005
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 17068
Application Number = 78474
Date of Application = 6,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 17069
Application Number = 78474
Date of Application = 6,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 17083
Application Number = 78516
Date of Application = 1,, May, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 17084
Application Number = 78516
Date of Application = 1,, May, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = ZYDUS PHARMS USA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27626
Application Number = 78528
Date of Application = 16,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 27627
Application Number = 78528
Date of Application = 16,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 27628
Application Number = 78528
Date of Application = 16,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 27629
Application Number = 78528
Date of Application = 16,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27630
Application Number = 78528
Date of Application = 16,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 005
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 27631
Application Number = 78528
Date of Application = 16,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 006
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 27656
Application Number = 78707
Date of Application = 29,, Dec, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 27657
Application Number = 78707
Date of Application = 29,, Dec, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 27658
Application Number = 78707
Date of Application = 29,, Dec, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 27659
Application Number = 78707
Date of Application = 29,, Dec, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 004
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27660
Application Number = 78707
Date of Application = 29,, Dec, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 005
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 27661
Application Number = 78707
Date of Application = 29,, Dec, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = VINTAGE
ProductNo = 006
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 27668
Application Number = 78740
Date of Application = 29,, May, 2009
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WEST WARD PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27669
Application Number = 78740
Date of Application = 29,, May, 2009
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WEST WARD PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 27670
Application Number = 78740
Date of Application = 29,, May, 2009
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WEST WARD PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 1MG
----
Product id = 27671
Application Number = 78740
Date of Application = 29,, May, 2009
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WEST WARD PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = 2MG
----
Product id = 27672
Application Number = 78740
Date of Application = 29,, May, 2009
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WEST WARD PHARMS
ProductNo = 005
Tecode =
Rld = No
Strength = 3MG
----
Product id = 27673
Application Number = 78740
Date of Application = 29,, May, 2009
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WEST WARD PHARMS
ProductNo = 006
Tecode =
Rld = No
Strength = 4MG
----
Product id = 14033
Application Number = 78744
Date of Application = 8,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1MG/ML
----
Product id = 27578
Application Number = 78828
Date of Application = 23,, Mar, 2009
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CADISTA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.25MG
----
Product id = 27579
Application Number = 78828
Date of Application = 23,, Mar, 2009
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CADISTA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 0.5MG
----
Product id = 27580
Application Number = 78828
Date of Application = 23,, Mar, 2009
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CADISTA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 1MG
----
Product id = 27581
Application Number = 78828
Date of Application = 23,, Mar, 2009
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CADISTA PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = 2MG
----
Product id = 27582
Application Number = 78828
Date of Application = 23,, Mar, 2009
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CADISTA PHARMS
ProductNo = 005
Tecode =
Rld = No
Strength = 3MG
----
Product id = 27583
Application Number = 78828
Date of Application = 23,, Mar, 2009
RX/OTC/DISCN = DISCN
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CADISTA PHARMS
ProductNo = 006
Tecode =
Rld = No
Strength = 4MG
----
Product id = 27674
Application Number = 78871
Date of Application = 9,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 27675
Application Number = 78871
Date of Application = 9,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 27676
Application Number = 78871
Date of Application = 9,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 27677
Application Number = 78871
Date of Application = 9,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 004
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27678
Application Number = 78871
Date of Application = 9,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 005
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 27679
Application Number = 78871
Date of Application = 9,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 006
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 14026
Application Number = 78909
Date of Application = 29,, Jul, 2009
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = BIO PHARM INC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1MG/ML
----
Product id = 14031
Application Number = 79059
Date of Application = 12,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = TRIS PHARMA INC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1MG/ML
----
Product id = 27650
Application Number = 79088
Date of Application = 30,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 27651
Application Number = 79088
Date of Application = 30,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 27652
Application Number = 79088
Date of Application = 30,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 27653
Application Number = 79088
Date of Application = 30,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27654
Application Number = 79088
Date of Application = 30,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 005
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 27655
Application Number = 79088
Date of Application = 30,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS
ProductNo = 006
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 14032
Application Number = 79158
Date of Application = 3,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = VINTAGE
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1MG/ML
----
Product id = 14030
Application Number = 90347
Date of Application = 7,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1MG/ML
----
Product id = 17048
Application Number = 90839
Date of Application = 4,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = JUBILANT GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 17049
Application Number = 90839
Date of Application = 4,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = JUBILANT GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 17050
Application Number = 90839
Date of Application = 4,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = JUBILANT GENERICS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 17051
Application Number = 90839
Date of Application = 4,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = JUBILANT GENERICS
ProductNo = 004
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 17052
Application Number = 90839
Date of Application = 4,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = JUBILANT GENERICS
ProductNo = 005
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 14022
Application Number = 91384
Date of Application = 25,, May, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1MG/ML
----
Product id = 17054
Application Number = 91537
Date of Application = 30,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 17055
Application Number = 91537
Date of Application = 30,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 17056
Application Number = 91537
Date of Application = 30,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 17057
Application Number = 91537
Date of Application = 30,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 17058
Application Number = 91537
Date of Application = 30,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 17053
Application Number = 91537
Date of Application = 12,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 006
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 27560
Application Number = 201003
Date of Application = 24,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AJANTA PHARMA LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.25MG
----
Product id = 27561
Application Number = 201003
Date of Application = 24,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AJANTA PHARMA LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.5MG
----
Product id = 27562
Application Number = 201003
Date of Application = 24,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AJANTA PHARMA LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 1MG
----
Product id = 27563
Application Number = 201003
Date of Application = 24,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AJANTA PHARMA LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = 2MG
----
Product id = 27564
Application Number = 201003
Date of Application = 24,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AJANTA PHARMA LTD
ProductNo = 005
Tecode = AB
Rld = No
Strength = 3MG
----
Product id = 27565
Application Number = 201003
Date of Application = 24,, Aug, 2011
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AJANTA PHARMA LTD
ProductNo = 006
Tecode = AB
Rld = No
Strength = 4MG
----
Product id = 14029
Application Number = 202386
Date of Application = 12,, Jan, 2015
RX/OTC/DISCN = RX
Tradename = RISPERIDONE
Route/format = ORAL / SOLUTION
Application Type = A
Applicant Name = SILARX PHARMS INC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 1MG/ML
----