sildenafil-citrate
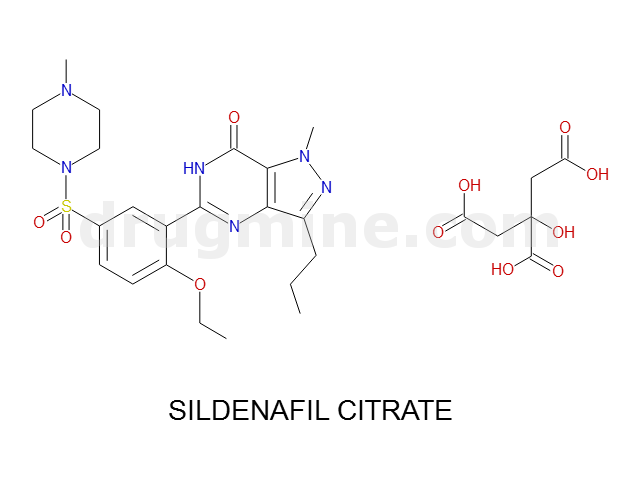
Name: SILDENAFIL CITRATE
ID :
MW: 475
Number of atoms: 33
Molecular_Formula: C22H30N6O4S
Alogp: 2.247
Indication class : Impotence Therapy
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
sildenafil citrate containing products summary
There are in total 16 different products containing the active ingredient sildenafil citrate. From the 16 drug products, zero have been discontinued.Product id = 29571
Application Number = 20895
Date of Application = 27,, Mar, 1998
RX/OTC/DISCN = RX
Tradename = VIAGRA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER IRELAND
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 25MG BASE
----
Product id = 29572
Application Number = 20895
Date of Application = 27,, Mar, 1998
RX/OTC/DISCN = RX
Tradename = VIAGRA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER IRELAND
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 50MG BASE
----
Product id = 29573
Application Number = 20895
Date of Application = 27,, Mar, 1998
RX/OTC/DISCN = RX
Tradename = VIAGRA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER IRELAND
ProductNo = 003
Tecode =
Rld = Yes
Strength = EQ 100MG BASE
----
Product id = 27512
Application Number = 21845
Date of Application = 3,, Jun, 2005
RX/OTC/DISCN = RX
Tradename = REVATIO
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 20MG BASE
----
Product id = 13583
Application Number = 22473
Date of Application = 18,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = REVATIO
Route/format = INTRAVENOUS / SOLUTION
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 10MG BASE/12.5ML (EQ 0.8MG BASE/ML)
----
Product id = 27995
Application Number = 78380
Date of Application = 7,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = SILDENAFIL CITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27990
Application Number = 91379
Date of Application = 6,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = SILDENAFIL CITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX CORP
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27996
Application Number = 91479
Date of Application = 6,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = SILDENAFIL CITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27988
Application Number = 200149
Date of Application = 25,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = SILDENAFIL CITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS GRP PTC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27994
Application Number = 201150
Date of Application = 9,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = SILDENAFIL CITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27989
Application Number = 202025
Date of Application = 28,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = SILDENAFIL CITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27997
Application Number = 202503
Date of Application = 6,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = SILDENAFIL CITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27991
Application Number = 202598
Date of Application = 6,, Nov, 2012
RX/OTC/DISCN = RX
Tradename = SILDENAFIL CITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 5270
Application Number = 203109
Date of Application = 30,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = REVATIO
Route/format = ORAL / FOR SUSPENSION
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 10MG BASE/ML
----
Product id = 27992
Application Number = 203623
Date of Application = 26,, Nov, 2014
RX/OTC/DISCN = RX
Tradename = SILDENAFIL CITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HETERO LABS LTD V
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 27993
Application Number = 203814
Date of Application = 17,, Dec, 2013
RX/OTC/DISCN = RX
Tradename = SILDENAFIL CITRATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MACLEODS PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----