telmisartan
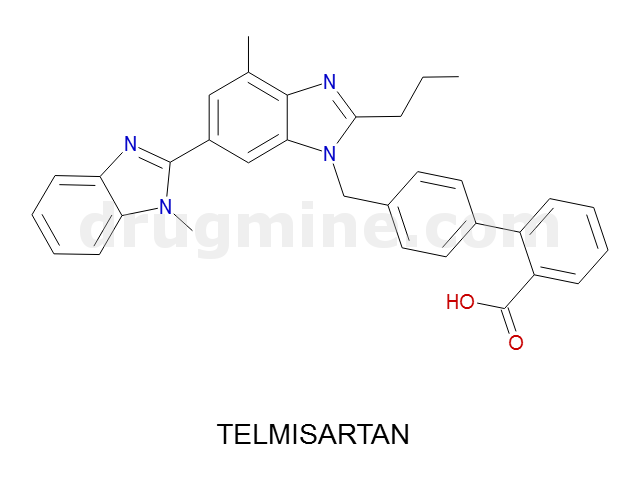


Name: TELMISARTAN
ID :
MW: 515
Number of atoms: 39
Molecular_Formula: C33H30N4O2
Alogp: 7.799
Indication class : Antagonist (angiotensin II receptor); Antihypertensive
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
telmisartan containing products summary
There are in total 55 different products containing the active ingredient telmisartan. From the 55 drug products, zero have been discontinued.Product id = 24908
Application Number = 20850
Date of Application = 10,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = MICARDIS
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 24909
Application Number = 20850
Date of Application = 10,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = MICARDIS
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 80MG
----
Product id = 24907
Application Number = 20850
Date of Application = 4,, Apr, 2000
RX/OTC/DISCN = RX
Tradename = MICARDIS
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 003
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 24910
Application Number = 21162
Date of Application = 17,, Nov, 2000
RX/OTC/DISCN = RX
Tradename = MICARDIS HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;40MG
----
Product id = 24911
Application Number = 21162
Date of Application = 17,, Nov, 2000
RX/OTC/DISCN = RX
Tradename = MICARDIS HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;80MG
----
Product id = 24912
Application Number = 21162
Date of Application = 19,, Apr, 2004
RX/OTC/DISCN = RX
Tradename = MICARDIS HCT
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 25MG;80MG
----
Product id = 29259
Application Number = 22401
Date of Application = 16,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = TWYNSTA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;40MG
----
Product id = 29261
Application Number = 22401
Date of Application = 16,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = TWYNSTA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;40MG
----
Product id = 29260
Application Number = 22401
Date of Application = 16,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = TWYNSTA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;80MG
----
Product id = 29262
Application Number = 22401
Date of Application = 16,, Oct, 2009
RX/OTC/DISCN = RX
Tradename = TWYNSTA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 004
Tecode = AB
Rld = Yes
Strength = EQ 10MG BASE;80MG
----
Product id = 28517
Application Number = 78710
Date of Application = 8,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 28518
Application Number = 78710
Date of Application = 8,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 28519
Application Number = 78710
Date of Application = 8,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 28505
Application Number = 90032
Date of Application = 7,, Jul, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 28506
Application Number = 90032
Date of Application = 7,, Jul, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 28507
Application Number = 90032
Date of Application = 7,, Jul, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 28538
Application Number = 91351
Date of Application = 7,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;40MG
----
Product id = 28539
Application Number = 91351
Date of Application = 7,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;80MG
----
Product id = 28540
Application Number = 91351
Date of Application = 7,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;80MG
----
Product id = 28541
Application Number = 91648
Date of Application = 25,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;40MG
----
Product id = 28542
Application Number = 91648
Date of Application = 25,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;80MG
----
Product id = 28543
Application Number = 91648
Date of Application = 25,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;80MG
----
Product id = 28544
Application Number = 201192
Date of Application = 25,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;40MG
----
Product id = 28545
Application Number = 201192
Date of Application = 25,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;80MG
----
Product id = 28546
Application Number = 201192
Date of Application = 25,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;80MG
----
Product id = 28523
Application Number = 201586
Date of Application = 8,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND AMLODIPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;40MG
----
Product id = 28525
Application Number = 201586
Date of Application = 8,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND AMLODIPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;40MG
----
Product id = 28524
Application Number = 201586
Date of Application = 8,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND AMLODIPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;80MG
----
Product id = 28526
Application Number = 201586
Date of Application = 8,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND AMLODIPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;80MG
----
Product id = 28502
Application Number = 202130
Date of Application = 7,, Jul, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 28503
Application Number = 202130
Date of Application = 7,, Jul, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 28504
Application Number = 202130
Date of Application = 7,, Jul, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 28508
Application Number = 202397
Date of Application = 7,, Jul, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 28509
Application Number = 202397
Date of Application = 7,, Jul, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 28510
Application Number = 202397
Date of Application = 7,, Jul, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 28527
Application Number = 202516
Date of Application = 26,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND AMLODIPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;40MG
----
Product id = 28529
Application Number = 202516
Date of Application = 26,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND AMLODIPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;40MG
----
Product id = 28528
Application Number = 202516
Date of Application = 26,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND AMLODIPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;80MG
----
Product id = 28530
Application Number = 202516
Date of Application = 26,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND AMLODIPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;80MG
----
Product id = 28531
Application Number = 202517
Date of Application = 8,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND AMLODIPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;40MG
----
Product id = 28533
Application Number = 202517
Date of Application = 8,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND AMLODIPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;40MG
----
Product id = 28532
Application Number = 202517
Date of Application = 8,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND AMLODIPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;80MG
----
Product id = 28534
Application Number = 202517
Date of Application = 8,, Jan, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND AMLODIPINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;80MG
----
Product id = 28535
Application Number = 203010
Date of Application = 25,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 12.5MG;40MG
----
Product id = 28536
Application Number = 203010
Date of Application = 25,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 12.5MG;80MG
----
Product id = 28537
Application Number = 203010
Date of Application = 25,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ALEMBIC PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 25MG;80MG
----
Product id = 28514
Application Number = 203171
Date of Application = 7,, Jul, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 28515
Application Number = 203171
Date of Application = 7,, Jul, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 28516
Application Number = 203171
Date of Application = 7,, Jul, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TORRENT PHARMS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 28520
Application Number = 203325
Date of Application = 26,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 28521
Application Number = 203325
Date of Application = 26,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 28522
Application Number = 203325
Date of Application = 26,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 80MG
----
Product id = 28511
Application Number = 203867
Date of Application = 3,, Nov, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 20MG
----
Product id = 28512
Application Number = 203867
Date of Application = 3,, Nov, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 40MG
----
Product id = 28513
Application Number = 203867
Date of Application = 3,, Nov, 2014
RX/OTC/DISCN = RX
Tradename = TELMISARTAN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 80MG
----