tolmetin-sodium
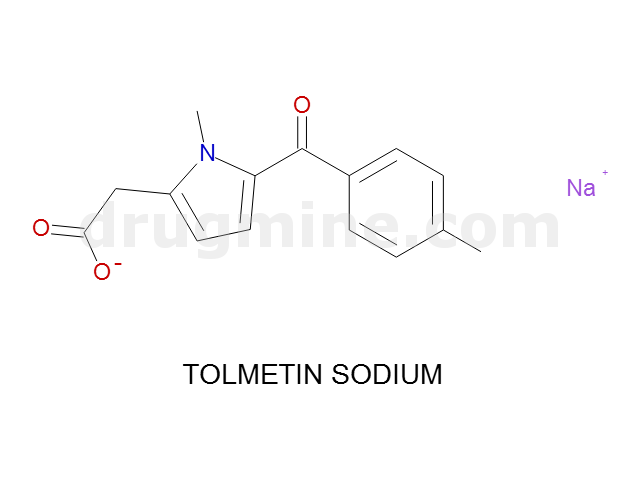
Name: TOLMETIN SODIUM
ID :
MW: 256
Number of atoms: 19
Molecular_Formula: C15H14NO3
Alogp: 1.793
Indication class : Anti-Inflammatory
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
tolmetin sodium containing products summary
There are in total 17 different products containing the active ingredient tolmetin sodium. From the 17 drug products, 13 have been discontinued.Product id = 28826
Application Number = 17628
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TOLECTIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ORTHO MCNEIL JANSSEN
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 200MG BASE
----
Product id = 28827
Application Number = 17628
Date of Application = 8,, Mar, 1989
RX/OTC/DISCN = DISCN
Tradename = TOLECTIN 600
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ORTHO MCNEIL JANSSEN
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 600MG BASE
----
Product id = 3604
Application Number = 18084
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TOLECTIN DS
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ORTHO MCNEIL JANSSEN
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 400MG BASE
----
Product id = 3610
Application Number = 73290
Date of Application = 27,, Nov, 1991
RX/OTC/DISCN = RX
Tradename = TOLMETIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 400MG BASE
----
Product id = 3605
Application Number = 73308
Date of Application = 24,, Jan, 1992
RX/OTC/DISCN = DISCN
Tradename = TOLMETIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 400MG BASE
----
Product id = 28833
Application Number = 73310
Date of Application = 27,, Nov, 1991
RX/OTC/DISCN = RX
Tradename = TOLMETIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 200MG BASE
----
Product id = 3607
Application Number = 73311
Date of Application = 27,, Nov, 1991
RX/OTC/DISCN = DISCN
Tradename = TOLMETIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 400MG BASE
----
Product id = 3606
Application Number = 73392
Date of Application = 24,, Jan, 1992
RX/OTC/DISCN = DISCN
Tradename = TOLMETIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 400MG BASE
----
Product id = 3608
Application Number = 73393
Date of Application = 27,, May, 1993
RX/OTC/DISCN = RX
Tradename = TOLMETIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 400MG BASE
----
Product id = 3609
Application Number = 73462
Date of Application = 30,, Apr, 1992
RX/OTC/DISCN = DISCN
Tradename = TOLMETIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 400MG BASE
----
Product id = 3611
Application Number = 73519
Date of Application = 29,, May, 1992
RX/OTC/DISCN = DISCN
Tradename = TOLMETIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 400MG BASE
----
Product id = 28831
Application Number = 73527
Date of Application = 30,, Jun, 1992
RX/OTC/DISCN = DISCN
Tradename = TOLMETIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 600MG BASE
----
Product id = 28835
Application Number = 73588
Date of Application = 31,, Jul, 1992
RX/OTC/DISCN = DISCN
Tradename = TOLMETIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 200MG BASE
----
Product id = 28836
Application Number = 74002
Date of Application = 27,, Sep, 1993
RX/OTC/DISCN = DISCN
Tradename = TOLMETIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 600MG BASE
----
Product id = 28832
Application Number = 74399
Date of Application = 28,, Mar, 1996
RX/OTC/DISCN = DISCN
Tradename = TOLMETIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 600MG BASE
----
Product id = 28834
Application Number = 74473
Date of Application = 30,, Aug, 1994
RX/OTC/DISCN = RX
Tradename = TOLMETIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 600MG BASE
----
Product id = 28837
Application Number = 74729
Date of Application = 27,, Feb, 1997
RX/OTC/DISCN = DISCN
Tradename = TOLMETIN SODIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 600MG BASE
----