topotecan-hydrochloride
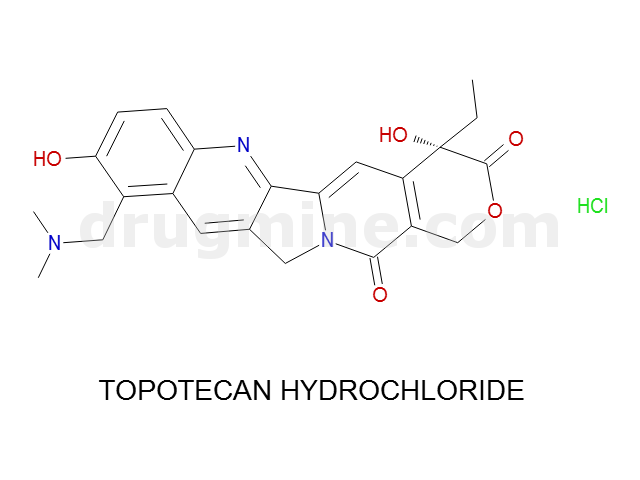


Name: TOPOTECAN HYDROCHLORIDE
ID :
MW: 421
Number of atoms: 31
Molecular_Formula: C23H23N3O5
Alogp: 1.577
Indication class : Antineoplastic (DNA topoisomerase I Inhibitor)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
topotecan hydrochloride containing products summary
There are in total 18 different products containing the active ingredient topotecan hydrochloride. From the 18 drug products, 4 have been discontinued.Product id = 8261
Application Number = 20671
Date of Application = 28,, May, 1996
RX/OTC/DISCN = RX
Tradename = HYCAMTIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 4MG BASE/VIAL
----
Product id = 2135
Application Number = 20981
Date of Application = 11,, Oct, 2007
RX/OTC/DISCN = RX
Tradename = HYCAMTIN
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = GLAXO WELLCOME
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.25MG BASE
----
Product id = 2136
Application Number = 20981
Date of Application = 11,, Oct, 2007
RX/OTC/DISCN = RX
Tradename = HYCAMTIN
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = GLAXO WELLCOME
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 1MG BASE
----
Product id = 13593
Application Number = 22453
Date of Application = 20,, Dec, 2012
RX/OTC/DISCN = RX
Tradename = TOPOTECAN HYDROCHLORIDE
Route/format = INTRAVENOUS / SOLUTION
Application Type = N
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/4ML (EQ 1MG BASE/ML)
----
Product id = 11126
Application Number = 90620
Date of Application = 2,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = TOPOTECAN HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACTAVIS TOTOWA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/VIAL
----
Product id = 11130
Application Number = 91089
Date of Application = 29,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = TOPOTECAN HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/VIAL
----
Product id = 11127
Application Number = 91199
Date of Application = 1,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = TOPOTECAN HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/VIAL
----
Product id = 11133
Application Number = 91284
Date of Application = 26,, Jan, 2011
RX/OTC/DISCN = RX
Tradename = TOPOTECAN HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT PHARMS
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/VIAL
----
Product id = 11129
Application Number = 91376
Date of Application = 29,, Nov, 2010
RX/OTC/DISCN = DISCN
Tradename = TOPOTECAN HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI ONCOL
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE/VIAL
----
Product id = 11132
Application Number = 91542
Date of Application = 28,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = TOPOTECAN HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ONCO THERAPIES LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/VIAL
----
Product id = 13589
Application Number = 200199
Date of Application = 25,, Feb, 2011
RX/OTC/DISCN = DISCN
Tradename = TOPOTECAN
Route/format = INTRAVENOUS / SOLUTION
Application Type = N
Applicant Name = SANDOZ INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1MG BASE/ML (EQ 1MG BASE/ML)
----
Product id = 13590
Application Number = 200199
Date of Application = 25,, Feb, 2011
RX/OTC/DISCN = DISCN
Tradename = TOPOTECAN
Route/format = INTRAVENOUS / SOLUTION
Application Type = N
Applicant Name = SANDOZ INC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 3MG BASE/3ML (EQ 1MG BASE/ML)
----
Product id = 13591
Application Number = 200199
Date of Application = 25,, Feb, 2011
RX/OTC/DISCN = DISCN
Tradename = TOPOTECAN
Route/format = INTRAVENOUS / SOLUTION
Application Type = N
Applicant Name = SANDOZ INC
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 4MG BASE/4ML (EQ 1MG BASE/ML)
----
Product id = 13592
Application Number = 200582
Date of Application = 2,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = TOPOTECAN HYDROCHLORIDE
Route/format = INTRAVENOUS / SOLUTION
Application Type = N
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 4MG BASE/4ML (EQ 1MG BASE/ML)
----
Product id = 11131
Application Number = 201166
Date of Application = 8,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = TOPOTECAN HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = INNOPHARMA LICENSING
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/VIAL
----
Product id = 11128
Application Number = 201191
Date of Application = 9,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = TOPOTECAN HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/VIAL
----
Product id = 11134
Application Number = 202203
Date of Application = 29,, Aug, 2013
RX/OTC/DISCN = RX
Tradename = TOPOTECAN HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SUN PHARM INDS LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/VIAL
----
Product id = 11125
Application Number = 202351
Date of Application = 26,, Jun, 2013
RX/OTC/DISCN = RX
Tradename = TOPOTECAN HYDROCHLORIDE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 4MG BASE/VIAL
----