valproic-acid
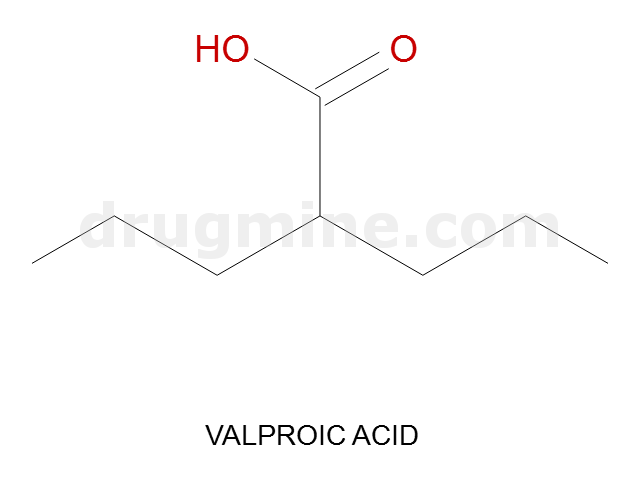


Name: VALPROIC ACID
ID :
MW: 144
Number of atoms: 10
Molecular_Formula: C8H16O2
Alogp: 2.749
Indication class : Anticonvulsant
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
valproic acid containing products summary
There are in total 19 different products containing the active ingredient valproic acid. From the 19 drug products, 7 have been discontinued.Product id = 1588
Application Number = 18081
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DEPAKENE
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = ABBVIE
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 250MG
----
Product id = 14981
Application Number = 18082
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = DEPAKENE
Route/format = ORAL / SYRUP
Application Type = N
Applicant Name = ABBVIE
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = 250MG/5ML
----
Product id = 281
Application Number = 22152
Date of Application = 29,, Jul, 2008
RX/OTC/DISCN = DISCN
Tradename = STAVZOR
Route/format = ORAL / CAPSULE, DELAYED RELEASE
Application Type = N
Applicant Name = BANNER LIFE SCIENCES
ProductNo = 001
Tecode =
Rld = No
Strength = 125MG
----
Product id = 282
Application Number = 22152
Date of Application = 29,, Jul, 2008
RX/OTC/DISCN = DISCN
Tradename = STAVZOR
Route/format = ORAL / CAPSULE, DELAYED RELEASE
Application Type = N
Applicant Name = BANNER LIFE SCIENCES
ProductNo = 002
Tecode =
Rld = No
Strength = 250MG
----
Product id = 283
Application Number = 22152
Date of Application = 29,, Jul, 2008
RX/OTC/DISCN = DISCN
Tradename = STAVZOR
Route/format = ORAL / CAPSULE, DELAYED RELEASE
Application Type = N
Applicant Name = BANNER LIFE SCIENCES
ProductNo = 003
Tecode =
Rld = No
Strength = 500MG
----
Product id = 3692
Application Number = 70195
Date of Application = 2,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = VALPROIC ACID
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SCHERER RP
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 3691
Application Number = 70431
Date of Application = 28,, Feb, 1986
RX/OTC/DISCN = DISCN
Tradename = VALPROIC ACID
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = PAR PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 3694
Application Number = 70631
Date of Application = 11,, Jun, 1987
RX/OTC/DISCN = DISCN
Tradename = VALPROIC ACID
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = USL PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG
----
Product id = 15177
Application Number = 70868
Date of Application = 1,, Jul, 1986
RX/OTC/DISCN = RX
Tradename = VALPROIC ACID
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AA
Rld = No
Strength = 250MG/5ML
----
Product id = 15171
Application Number = 73178
Date of Application = 25,, Aug, 1992
RX/OTC/DISCN = RX
Tradename = VALPROIC ACID
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = ANI PHARMS INC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 250MG/5ML
----
Product id = 3690
Application Number = 73229
Date of Application = 29,, Oct, 1991
RX/OTC/DISCN = RX
Tradename = VALPROIC ACID
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = CATALENT
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 3689
Application Number = 73484
Date of Application = 29,, Jun, 1993
RX/OTC/DISCN = RX
Tradename = VALPROIC ACID
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = BANNER LIFE SCIENCES
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----
Product id = 15173
Application Number = 74060
Date of Application = 13,, Jan, 1995
RX/OTC/DISCN = RX
Tradename = VALPROIC ACID
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = HIGH TECH PHARMA
ProductNo = 001
Tecode = AA
Rld = No
Strength = 250MG/5ML
----
Product id = 15174
Application Number = 75379
Date of Application = 15,, Dec, 2000
RX/OTC/DISCN = RX
Tradename = VALPROIC ACID
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = PHARM ASSOC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 250MG/5ML
----
Product id = 15170
Application Number = 75782
Date of Application = 22,, Dec, 2000
RX/OTC/DISCN = RX
Tradename = VALPROIC ACID
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = ALPHARMA
ProductNo = 001
Tecode = AA
Rld = No
Strength = 250MG/5ML
----
Product id = 15172
Application Number = 77105
Date of Application = 29,, Jul, 2005
RX/OTC/DISCN = DISCN
Tradename = VALPROIC ACID
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 250MG/5ML
----
Product id = 15176
Application Number = 77960
Date of Application = 13,, Oct, 2006
RX/OTC/DISCN = RX
Tradename = VALPROIC ACID
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = VINTAGE
ProductNo = 001
Tecode = AA
Rld = No
Strength = 250MG/5ML
----
Product id = 15175
Application Number = 90517
Date of Application = 28,, May, 2010
RX/OTC/DISCN = RX
Tradename = VALPROIC ACID
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = SUN PHARM INDS INC
ProductNo = 001
Tecode = AA
Rld = No
Strength = 250MG/5ML
----
Product id = 3693
Application Number = 91037
Date of Application = 22,, Feb, 2013
RX/OTC/DISCN = RX
Tradename = VALPROIC ACID
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = SUN PHARM INDS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 250MG
----