albuterol-sulfate
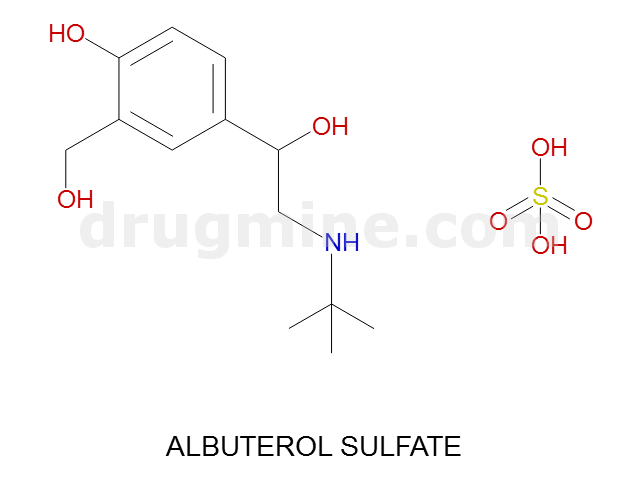

Name: ALBUTEROL SULFATE
ID :
MW: 239
Number of atoms: 17
Molecular_Formula: C13H21NO3
Alogp: 0.941
Indication class : Bronchodilator
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
albuterol sulfate containing products summary
There are in total 94 different products containing the active ingredient albuterol sulfate. From the 94 drug products, 53 have been discontinued.Product id = 27054
Application Number = 17853
Date of Application = 7,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = PROVENTIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 27055
Application Number = 17853
Date of Application = 7,, May, 1982
RX/OTC/DISCN = DISCN
Tradename = PROVENTIL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = SCHERING
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 15126
Application Number = 18062
Date of Application = 19,, Jan, 1983
RX/OTC/DISCN = DISCN
Tradename = PROVENTIL
Route/format = ORAL / SYRUP
Application Type = N
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/5ML
----
Product id = 29530
Application Number = 19112
Date of Application = 10,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = VENTOLIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 29531
Application Number = 19112
Date of Application = 10,, Jul, 1986
RX/OTC/DISCN = DISCN
Tradename = VENTOLIN
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 13402
Application Number = 19243
Date of Application = 14,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = PROVENTIL
Route/format = INHALATION / SOLUTION
Application Type = N
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.5% BASE
----
Product id = 13401
Application Number = 19243
Date of Application = 14,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = PROVENTIL
Route/format = INHALATION / SOLUTION
Application Type = N
Applicant Name = SCHERING
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 0.083% BASE
----
Product id = 13414
Application Number = 19269
Date of Application = 16,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = VENTOLIN
Route/format = INHALATION / SOLUTION
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 0.5% BASE
----
Product id = 16551
Application Number = 19383
Date of Application = 13,, Jul, 1987
RX/OTC/DISCN = DISCN
Tradename = PROVENTIL
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 878
Application Number = 19489
Date of Application = 4,, May, 1988
RX/OTC/DISCN = DISCN
Tradename = VENTOLIN ROTACAPS
Route/format = INHALATION / CAPSULE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.2MG BASE
----
Product id = 16774
Application Number = 19604
Date of Application = 23,, Dec, 1992
RX/OTC/DISCN = DISCN
Tradename = VOLMAX
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = MURO
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 8MG BASE
----
Product id = 16773
Application Number = 19604
Date of Application = 23,, Dec, 1992
RX/OTC/DISCN = DISCN
Tradename = VOLMAX
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = MURO
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 15178
Application Number = 19621
Date of Application = 10,, Jun, 1987
RX/OTC/DISCN = DISCN
Tradename = VENTOLIN
Route/format = ORAL / SYRUP
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/5ML
----
Product id = 13413
Application Number = 19773
Date of Application = 23,, Apr, 1992
RX/OTC/DISCN = DISCN
Tradename = VENTOLIN
Route/format = INHALATION / SOLUTION
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.083% BASE
----
Product id = 44
Application Number = 20291
Date of Application = 24,, Oct, 1996
RX/OTC/DISCN = RX
Tradename = COMBIVENT
Route/format = INHALATION / AEROSOL, METERED
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 0.09MG BASE/INH;0.018MG/INH
----
Product id = 69
Application Number = 20503
Date of Application = 15,, Aug, 1996
RX/OTC/DISCN = RX
Tradename = PROVENTIL-HFA
Route/format = INHALATION / AEROSOL, METERED
Application Type = N
Applicant Name = 3M
ProductNo = 001
Tecode = BX
Rld = Yes
Strength = EQ 0.09MG BASE/INH
----
Product id = 13258
Application Number = 20949
Date of Application = 30,, Apr, 2001
RX/OTC/DISCN = RX
Tradename = ACCUNEB
Route/format = INHALATION / SOLUTION
Application Type = N
Applicant Name = MYLAN SPECLT
ProductNo = 001
Tecode = AN
Rld = Yes
Strength = EQ 0.042% BASE
----
Product id = 13257
Application Number = 20949
Date of Application = 30,, Apr, 2001
RX/OTC/DISCN = RX
Tradename = ACCUNEB
Route/format = INHALATION / SOLUTION
Application Type = N
Applicant Name = MYLAN SPECLT
ProductNo = 002
Tecode = AN
Rld = Yes
Strength = EQ 0.021% BASE
----
Product id = 13309
Application Number = 20950
Date of Application = 21,, Mar, 2001
RX/OTC/DISCN = RX
Tradename = DUONEB
Route/format = INHALATION / SOLUTION
Application Type = N
Applicant Name = MYLAN SPECLT
ProductNo = 001
Tecode = AN
Rld = Yes
Strength = EQ 0.083% BASE;0.017%
----
Product id = 80
Application Number = 20983
Date of Application = 19,, Apr, 2001
RX/OTC/DISCN = RX
Tradename = VENTOLIN HFA
Route/format = INHALATION / AEROSOL, METERED
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode = BX
Rld = Yes
Strength = EQ 0.09MG BASE/INH
----
Product id = 67
Application Number = 21457
Date of Application = 29,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = PROAIR HFA
Route/format = INHALATION / AEROSOL, METERED
Application Type = N
Applicant Name = TEVA BRANDED PHARM
ProductNo = 001
Tecode = BX
Rld = Yes
Strength = EQ 0.09MG BASE/INH
----
Product id = 14327
Application Number = 21747
Date of Application = 7,, Oct, 2011
RX/OTC/DISCN = RX
Tradename = COMBIVENT RESPIMAT
Route/format = INHALATION / SPRAY, METERED
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 0.1MG BASE/INH;0.02MG/INH
----
Product id = 17410
Application Number = 72151
Date of Application = 5,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 17411
Application Number = 72152
Date of Application = 5,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 17408
Application Number = 72316
Date of Application = 5,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 17409
Application Number = 72317
Date of Application = 5,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 17398
Application Number = 72449
Date of Application = 5,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AM THERAP
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 17399
Application Number = 72450
Date of Application = 5,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AM THERAP
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 17412
Application Number = 72619
Date of Application = 5,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 17415
Application Number = 72620
Date of Application = 5,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 17422
Application Number = 72629
Date of Application = 31,, Jan, 1991
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 17424
Application Number = 72630
Date of Application = 31,, Jan, 1991
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 17405
Application Number = 72637
Date of Application = 5,, Dec, 1989
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 17404
Application Number = 72637
Date of Application = 5,, Dec, 1989
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 13272
Application Number = 72652
Date of Application = 21,, Feb, 1992
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = MYLAN SPECLT
ProductNo = 001
Tecode = AN
Rld = Yes
Strength = EQ 0.083% BASE
----
Product id = 17423
Application Number = 72764
Date of Application = 28,, Aug, 1991
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 17425
Application Number = 72765
Date of Application = 28,, Aug, 1991
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 17413
Application Number = 72779
Date of Application = 25,, Jun, 1993
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 17416
Application Number = 72780
Date of Application = 25,, Jun, 1993
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 17420
Application Number = 72817
Date of Application = 9,, Jan, 1990
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 17421
Application Number = 72818
Date of Application = 9,, Jan, 1990
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WARNER CHILCOTT
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 17402
Application Number = 72859
Date of Application = 20,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 17403
Application Number = 72860
Date of Application = 20,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 17407
Application Number = 72894
Date of Application = 17,, Jan, 1991
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 4MG BASE
----
Product id = 17406
Application Number = 72894
Date of Application = 17,, Jan, 1991
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 2MG BASE
----
Product id = 17414
Application Number = 72938
Date of Application = 30,, Mar, 1990
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 17417
Application Number = 72939
Date of Application = 30,, Mar, 1990
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 17400
Application Number = 72966
Date of Application = 22,, Nov, 1991
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COPLEY PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 17401
Application Number = 72967
Date of Application = 22,, Nov, 1991
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = COPLEY PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 17418
Application Number = 73120
Date of Application = 29,, Sep, 1992
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UCB INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE
----
Product id = 17419
Application Number = 73121
Date of Application = 29,, Sep, 1992
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UCB INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 4MG BASE
----
Product id = 14885
Application Number = 73165
Date of Application = 29,, Apr, 1993
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/5ML
----
Product id = 13268
Application Number = 73307
Date of Application = 27,, Nov, 1991
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = COPLEY PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.5% BASE
----
Product id = 14882
Application Number = 73419
Date of Application = 30,, Mar, 1992
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AA
Rld = Yes
Strength = EQ 2MG BASE/5ML
----
Product id = 13267
Application Number = 73495
Date of Application = 28,, May, 1993
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = COPLEY PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.083% BASE
----
Product id = 13260
Application Number = 73533
Date of Application = 26,, Sep, 1995
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.083% BASE
----
Product id = 14881
Application Number = 74302
Date of Application = 30,, Sep, 1994
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = MOVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/5ML
----
Product id = 14877
Application Number = 74454
Date of Application = 25,, Sep, 1995
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 2MG BASE/5ML
----
Product id = 13270
Application Number = 74543
Date of Application = 15,, Jan, 1998
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = HI TECH PHARMA
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.5% BASE
----
Product id = 14880
Application Number = 74749
Date of Application = 30,, Jan, 1998
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = HI TECH PHARMA
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 2MG BASE/5ML
----
Product id = 13275
Application Number = 74880
Date of Application = 17,, Sep, 1997
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = NEPHRON
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.083% BASE
----
Product id = 13266
Application Number = 75050
Date of Application = 18,, Jun, 1998
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = BAUSCH AND LOMB
ProductNo = 001
Tecode = AN
Rld = Yes
Strength = EQ 0.5% BASE
----
Product id = 13269
Application Number = 75063
Date of Application = 9,, Feb, 1999
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = HI TECH PHARMA
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.083% BASE
----
Product id = 13278
Application Number = 75129
Date of Application = 13,, Feb, 2001
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = ROXANE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.083% BASE
----
Product id = 14878
Application Number = 75262
Date of Application = 30,, Mar, 1999
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2MG BASE/5ML
----
Product id = 13279
Application Number = 75343
Date of Application = 9,, Nov, 1999
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.083% BASE
----
Product id = 13265
Application Number = 75358
Date of Application = 29,, Mar, 2000
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = BAUSCH AND LOMB
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.083% BASE
----
Product id = 13283
Application Number = 75394
Date of Application = 22,, Nov, 1999
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = WOCKHARDT EU OPERATN
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.083% BASE
----
Product id = 13276
Application Number = 75664
Date of Application = 26,, Jun, 2001
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = NEPHRON
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.5% BASE
----
Product id = 13263
Application Number = 75717
Date of Application = 2,, Feb, 2007
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.083% BASE
----
Product id = 16776
Application Number = 76130
Date of Application = 26,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = VOSPIRE ER
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 16777
Application Number = 76130
Date of Application = 26,, Sep, 2002
RX/OTC/DISCN = RX
Tradename = VOSPIRE ER
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = DAVA PHARMS INC
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = EQ 8MG BASE
----
Product id = 13274
Application Number = 76355
Date of Application = 28,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = NEPHRON
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.042% BASE
----
Product id = 13273
Application Number = 76355
Date of Application = 31,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = NEPHRON
ProductNo = 002
Tecode = AN
Rld = No
Strength = EQ 0.021% BASE
----
Product id = 13282
Application Number = 76370
Date of Application = 24,, Nov, 2003
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.083% BASE
----
Product id = 13264
Application Number = 76391
Date of Application = 1,, Apr, 2003
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.5% BASE
----
Product id = 13289
Application Number = 76724
Date of Application = 31,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE AND IPRATROPIUM BROMIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.083% BASE;0.017%
----
Product id = 13286
Application Number = 76749
Date of Application = 31,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE AND IPRATROPIUM BROMIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = NEPHRON
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.083% BASE;0.017%
----
Product id = 13288
Application Number = 76867
Date of Application = 21,, Dec, 2006
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE AND IPRATROPIUM BROMIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.083% BASE;0.017%
----
Product id = 13290
Application Number = 77063
Date of Application = 31,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE AND IPRATROPIUM BROMIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.083% BASE;0.017%
----
Product id = 13284
Application Number = 77117
Date of Application = 31,, Dec, 2007
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE AND IPRATROPIUM BROMIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.083% BASE;0.017%
----
Product id = 13285
Application Number = 77559
Date of Application = 31,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE AND IPRATROPIUM BROMIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.083% BASE;0.017%
----
Product id = 13271
Application Number = 77569
Date of Application = 4,, Apr, 2006
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = LANDELA PHARM
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.083% BASE
----
Product id = 13280
Application Number = 77772
Date of Application = 25,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.021% BASE
----
Product id = 13281
Application Number = 77772
Date of Application = 25,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AN
Rld = No
Strength = EQ 0.042% BASE
----
Product id = 14884
Application Number = 77788
Date of Application = 26,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = VISTAPHARM
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 2MG BASE/5ML
----
Product id = 13277
Application Number = 77839
Date of Application = 16,, Dec, 2008
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = RITEDOSE CORP
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.083% BASE
----
Product id = 15641
Application Number = 78092
Date of Application = 29,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 8MG BASE
----
Product id = 15640
Application Number = 78092
Date of Application = 29,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 4MG BASE
----
Product id = 14883
Application Number = 78105
Date of Application = 27,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = VINTAGE
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 2MG BASE/5ML
----
Product id = 13261
Application Number = 78623
Date of Application = 5,, Apr, 2010
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.021% BASE
----
Product id = 13262
Application Number = 78623
Date of Application = 5,, Apr, 2010
RX/OTC/DISCN = DISCN
Tradename = ALBUTEROL SULFATE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 0.042% BASE
----
Product id = 14879
Application Number = 79241
Date of Application = 12,, May, 2010
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE
Route/format = ORAL / SYRUP
Application Type = A
Applicant Name = AMNEAL PHARMS
ProductNo = 001
Tecode = AA
Rld = No
Strength = EQ 2MG BASE/5ML
----
Product id = 13287
Application Number = 202496
Date of Application = 1,, Oct, 2012
RX/OTC/DISCN = RX
Tradename = ALBUTEROL SULFATE AND IPRATROPIUM BROMIDE
Route/format = INHALATION / SOLUTION
Application Type = A
Applicant Name = RITEDOSE CORP
ProductNo = 001
Tecode = AN
Rld = No
Strength = EQ 0.083% BASE;0.017%
----