atorvastatin-calcium

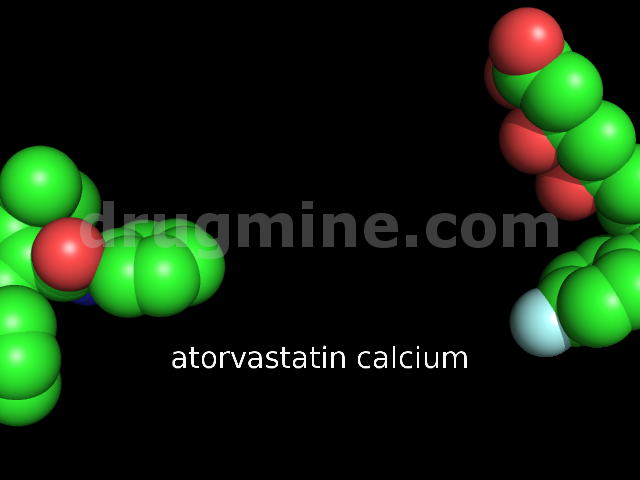
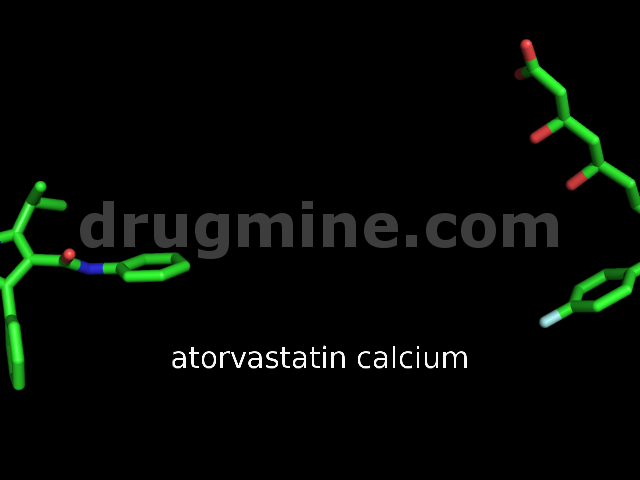
Name: ATORVASTATIN CALCIUM
ID :
MW: 558
Number of atoms: 41
Molecular_Formula: C33H34FN2O5
Alogp: 4.081
Indication class : Inhibitor (HMG-CoA reductase)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
atorvastatin calcium containing products summary
There are in total 69 different products containing the active ingredient atorvastatin calcium. From the 69 drug products, 4 have been discontinued.Product id = 23683
Application Number = 20702
Date of Application = 17,, Dec, 1996
RX/OTC/DISCN = RX
Tradename = LIPITOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 23684
Application Number = 20702
Date of Application = 17,, Dec, 1996
RX/OTC/DISCN = RX
Tradename = LIPITOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 23685
Application Number = 20702
Date of Application = 17,, Dec, 1996
RX/OTC/DISCN = RX
Tradename = LIPITOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 23686
Application Number = 20702
Date of Application = 7,, Apr, 2000
RX/OTC/DISCN = RX
Tradename = LIPITOR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 004
Tecode = AB
Rld = Yes
Strength = EQ 80MG BASE
----
Product id = 18775
Application Number = 21540
Date of Application = 30,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = CADUET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;EQ 10MG BASE
----
Product id = 18776
Application Number = 21540
Date of Application = 30,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = CADUET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;EQ 20MG BASE
----
Product id = 18777
Application Number = 21540
Date of Application = 30,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = CADUET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;EQ 40MG BASE
----
Product id = 18778
Application Number = 21540
Date of Application = 30,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = CADUET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;EQ 80MG BASE
----
Product id = 18779
Application Number = 21540
Date of Application = 30,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = CADUET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;EQ 10MG BASE
----
Product id = 18780
Application Number = 21540
Date of Application = 30,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = CADUET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;EQ 20MG BASE
----
Product id = 18781
Application Number = 21540
Date of Application = 30,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = CADUET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 007
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;EQ 40MG BASE
----
Product id = 18782
Application Number = 21540
Date of Application = 30,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = CADUET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 008
Tecode = AB
Rld = Yes
Strength = EQ 10MG BASE;EQ 80MG BASE
----
Product id = 18772
Application Number = 21540
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = CADUET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 009
Tecode = AB
Rld = No
Strength = EQ 2.5MG BASE;EQ 10MG BASE
----
Product id = 18773
Application Number = 21540
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = CADUET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 010
Tecode = AB
Rld = No
Strength = EQ 2.5MG BASE;EQ 20MG BASE
----
Product id = 18774
Application Number = 21540
Date of Application = 29,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = CADUET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 011
Tecode = AB
Rld = No
Strength = EQ 2.5MG BASE;EQ 40MG BASE
----
Product id = 18196
Application Number = 76477
Date of Application = 30,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 18197
Application Number = 76477
Date of Application = 30,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 18198
Application Number = 76477
Date of Application = 30,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 18199
Application Number = 76477
Date of Application = 30,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY LABS LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 80MG BASE
----
Product id = 18200
Application Number = 77575
Date of Application = 29,, May, 2012
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 18201
Application Number = 77575
Date of Application = 29,, May, 2012
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 18202
Application Number = 77575
Date of Application = 29,, May, 2012
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 18203
Application Number = 77575
Date of Application = 29,, May, 2012
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 80MG BASE
----
Product id = 18204
Application Number = 78773
Date of Application = 29,, May, 2012
RX/OTC/DISCN = DISCN
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE
----
Product id = 18205
Application Number = 78773
Date of Application = 29,, May, 2012
RX/OTC/DISCN = DISCN
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20MG BASE
----
Product id = 18206
Application Number = 78773
Date of Application = 29,, May, 2012
RX/OTC/DISCN = DISCN
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 40MG BASE
----
Product id = 18207
Application Number = 78773
Date of Application = 29,, May, 2012
RX/OTC/DISCN = DISCN
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 80MG BASE
----
Product id = 18180
Application Number = 90548
Date of Application = 29,, May, 2012
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 18181
Application Number = 90548
Date of Application = 29,, May, 2012
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 18182
Application Number = 90548
Date of Application = 29,, May, 2012
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 18183
Application Number = 90548
Date of Application = 29,, May, 2012
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 80MG BASE
----
Product id = 18192
Application Number = 91226
Date of Application = 29,, May, 2012
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 18193
Application Number = 91226
Date of Application = 29,, May, 2012
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 18194
Application Number = 91226
Date of Application = 29,, May, 2012
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 18195
Application Number = 91226
Date of Application = 29,, May, 2012
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 80MG BASE
----
Product id = 18188
Application Number = 91624
Date of Application = 5,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = KUDCO IRELAND
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 18189
Application Number = 91624
Date of Application = 5,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = KUDCO IRELAND
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 18190
Application Number = 91624
Date of Application = 5,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = KUDCO IRELAND
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 18191
Application Number = 91624
Date of Application = 5,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = KUDCO IRELAND
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 80MG BASE
----
Product id = 18184
Application Number = 91650
Date of Application = 17,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 10MG BASE
----
Product id = 18185
Application Number = 91650
Date of Application = 17,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 20MG BASE
----
Product id = 18186
Application Number = 91650
Date of Application = 17,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 40MG BASE
----
Product id = 23687
Application Number = 200153
Date of Application = 3,, May, 2013
RX/OTC/DISCN = RX
Tradename = LIPTRUZET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE;10MG
----
Product id = 23688
Application Number = 200153
Date of Application = 3,, May, 2013
RX/OTC/DISCN = RX
Tradename = LIPTRUZET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20MG BASE;10MG
----
Product id = 23689
Application Number = 200153
Date of Application = 3,, May, 2013
RX/OTC/DISCN = RX
Tradename = LIPTRUZET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 40MG BASE;10MG
----
Product id = 23690
Application Number = 200153
Date of Application = 3,, May, 2013
RX/OTC/DISCN = RX
Tradename = LIPTRUZET
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 004
Tecode =
Rld = Yes
Strength = EQ 80MG BASE;10MG
----
Product id = 17924
Application Number = 200465
Date of Application = 29,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2.5MG BASE;EQ 10MG BASE
----
Product id = 17925
Application Number = 200465
Date of Application = 29,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 2.5MG BASE;EQ 20MG BASE
----
Product id = 17926
Application Number = 200465
Date of Application = 29,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 2.5MG BASE;EQ 40MG BASE
----
Product id = 17927
Application Number = 200465
Date of Application = 29,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;EQ 10MG BASE
----
Product id = 17928
Application Number = 200465
Date of Application = 29,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;EQ 20MG BASE
----
Product id = 17929
Application Number = 200465
Date of Application = 29,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;EQ 40MG BASE
----
Product id = 17930
Application Number = 200465
Date of Application = 29,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 007
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;EQ 80MG BASE
----
Product id = 17931
Application Number = 200465
Date of Application = 29,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 008
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;EQ 10MG BASE
----
Product id = 17932
Application Number = 200465
Date of Application = 29,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 009
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;EQ 20MG BASE
----
Product id = 17933
Application Number = 200465
Date of Application = 29,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 010
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;EQ 40MG BASE
----
Product id = 17934
Application Number = 200465
Date of Application = 29,, Nov, 2013
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 011
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;EQ 80MG BASE
----
Product id = 18187
Application Number = 202357
Date of Application = 17,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 80MG BASE
----
Product id = 17913
Application Number = 203874
Date of Application = 7,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2.5MG BASE;EQ 10MG BASE
----
Product id = 17914
Application Number = 203874
Date of Application = 7,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 2.5MG BASE;EQ 20MG BASE
----
Product id = 17915
Application Number = 203874
Date of Application = 7,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 2.5MG BASE;EQ 40MG BASE
----
Product id = 17916
Application Number = 203874
Date of Application = 7,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;EQ 10MG BASE
----
Product id = 17917
Application Number = 203874
Date of Application = 7,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 005
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;EQ 20MG BASE
----
Product id = 17918
Application Number = 203874
Date of Application = 7,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 006
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;EQ 40MG BASE
----
Product id = 17919
Application Number = 203874
Date of Application = 7,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 007
Tecode = AB
Rld = No
Strength = EQ 5MG BASE;EQ 80MG BASE
----
Product id = 17920
Application Number = 203874
Date of Application = 7,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 008
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;EQ 10MG BASE
----
Product id = 17921
Application Number = 203874
Date of Application = 7,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 009
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;EQ 20MG BASE
----
Product id = 17922
Application Number = 203874
Date of Application = 7,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 010
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;EQ 40MG BASE
----
Product id = 17923
Application Number = 203874
Date of Application = 7,, Mar, 2014
RX/OTC/DISCN = RX
Tradename = AMLODIPINE BESYLATE AND ATORVASTATIN CALCIUM
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 011
Tecode = AB
Rld = No
Strength = EQ 10MG BASE;EQ 80MG BASE
----