azithromycin
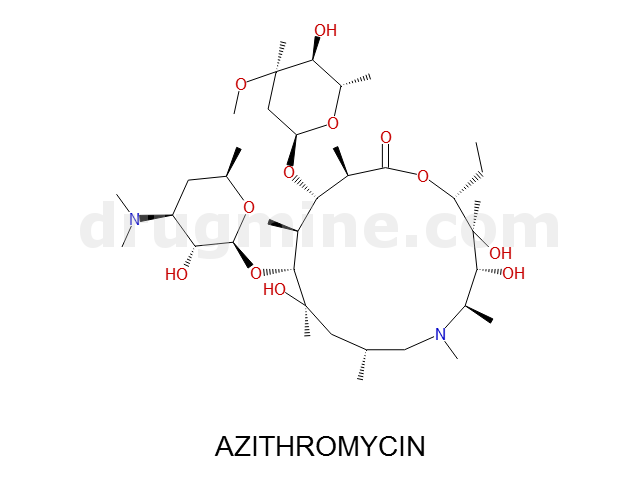

Name: AZITHROMYCIN
ID :
MW: 749
Number of atoms: 52
Molecular_Formula: C38H72N2O12
Alogp: 2.078
Indication class : Antibacterial
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
azithromycin containing products summary
There are in total 43 different products containing the active ingredient azithromycin. From the 43 drug products, 6 have been discontinued.Product id = 3846
Application Number = 50670
Date of Application = 1,, Nov, 1991
RX/OTC/DISCN = DISCN
Tradename = ZITHROMAX
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 5315
Application Number = 50693
Date of Application = 28,, Sep, 1994
RX/OTC/DISCN = RX
Tradename = ZITHROMAX
Route/format = ORAL / FOR SUSPENSION
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 1GM BASE/PACKET
----
Product id = 5316
Application Number = 50710
Date of Application = 19,, Oct, 1995
RX/OTC/DISCN = RX
Tradename = ZITHROMAX
Route/format = ORAL / FOR SUSPENSION
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 100MG BASE/5ML
----
Product id = 5317
Application Number = 50710
Date of Application = 19,, Oct, 1995
RX/OTC/DISCN = RX
Tradename = ZITHROMAX
Route/format = ORAL / FOR SUSPENSION
Application Type = N
Applicant Name = PFIZER
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = EQ 200MG BASE/5ML
----
Product id = 29794
Application Number = 50711
Date of Application = 18,, Jul, 1996
RX/OTC/DISCN = RX
Tradename = ZITHROMAX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 29796
Application Number = 50730
Date of Application = 12,, Jun, 1996
RX/OTC/DISCN = RX
Tradename = ZITHROMAX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 600MG BASE
----
Product id = 11485
Application Number = 50733
Date of Application = 30,, Jan, 1997
RX/OTC/DISCN = RX
Tradename = ZITHROMAX
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 500MG BASE/VIAL
----
Product id = 29795
Application Number = 50784
Date of Application = 24,, May, 2002
RX/OTC/DISCN = RX
Tradename = ZITHROMAX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = PFIZER
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 4980
Application Number = 50797
Date of Application = 10,, Jun, 2005
RX/OTC/DISCN = RX
Tradename = ZMAX
Route/format = ORAL / FOR SUSPENSION, EXTENDED RELEASE
Application Type = N
Applicant Name = PF PRISM CV
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 2GM BASE/BOT
----
Product id = 6063
Application Number = 50809
Date of Application = 19,, Dec, 2006
RX/OTC/DISCN = DISCN
Tradename = AZITHROMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = TEVA PARENTERAL
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6062
Application Number = 50809
Date of Application = 19,, Dec, 2006
RX/OTC/DISCN = DISCN
Tradename = AZITHROMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = TEVA PARENTERAL
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 2.5GM BASE/VIAL
----
Product id = 12862
Application Number = 50810
Date of Application = 27,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = AZASITE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = OAK PHARMS INC
ProductNo = 001
Tecode =
Rld = Yes
Strength = 1%
----
Product id = 18271
Application Number = 65150
Date of Application = 14,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 600MG BASE
----
Product id = 18269
Application Number = 65153
Date of Application = 14,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 6055
Application Number = 65179
Date of Application = 13,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 18270
Application Number = 65193
Date of Application = 14,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 18268
Application Number = 65209
Date of Application = 14,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 600MG BASE
----
Product id = 18266
Application Number = 65211
Date of Application = 14,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 18267
Application Number = 65212
Date of Application = 14,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 18265
Application Number = 65218
Date of Application = 14,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 600MG BASE
----
Product id = 18264
Application Number = 65223
Date of Application = 14,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 18263
Application Number = 65225
Date of Application = 14,, Nov, 2005
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 5061
Application Number = 65246
Date of Application = 5,, Jul, 2006
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 200MG BASE/5ML
----
Product id = 5060
Application Number = 65246
Date of Application = 5,, Jul, 2006
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = PLIVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 100MG BASE/5ML
----
Product id = 6059
Application Number = 65265
Date of Application = 18,, Jan, 2007
RX/OTC/DISCN = DISCN
Tradename = AZITHROMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = PLIVA HRVATSKA DOO
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 5062
Application Number = 65297
Date of Application = 18,, Sep, 2006
RX/OTC/DISCN = DISCN
Tradename = AZITHROMYCIN
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 100MG BASE/5ML
----
Product id = 5063
Application Number = 65297
Date of Application = 18,, Sep, 2006
RX/OTC/DISCN = DISCN
Tradename = AZITHROMYCIN
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 200MG BASE/5ML
----
Product id = 18274
Application Number = 65302
Date of Application = 11,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 600MG BASE
----
Product id = 18262
Application Number = 65360
Date of Application = 8,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 600MG BASE
----
Product id = 18260
Application Number = 65365
Date of Application = 30,, May, 2007
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 18261
Application Number = 65366
Date of Application = 30,, May, 2007
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 18272
Application Number = 65404
Date of Application = 11,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 18273
Application Number = 65405
Date of Application = 11,, Feb, 2008
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 5064
Application Number = 65419
Date of Application = 24,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 100MG BASE/5ML
----
Product id = 5065
Application Number = 65419
Date of Application = 24,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / FOR SUSPENSION
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 200MG BASE/5ML
----
Product id = 6057
Application Number = 65500
Date of Application = 26,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6056
Application Number = 65501
Date of Application = 9,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAND PHARMA LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6060
Application Number = 65506
Date of Application = 24,, Mar, 2009
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT STRIDES
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 18257
Application Number = 65507
Date of Application = 13,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX CORP
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 18259
Application Number = 65508
Date of Application = 13,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX CORP
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 600MG BASE
----
Product id = 18258
Application Number = 65509
Date of Application = 13,, Jul, 2011
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX CORP
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 6058
Application Number = 65511
Date of Application = 26,, Jun, 2009
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6061
Application Number = 90923
Date of Application = 2,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = AZITHROMYCIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SUN PHARM INDS LTD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----