betamethasone-dipropionate
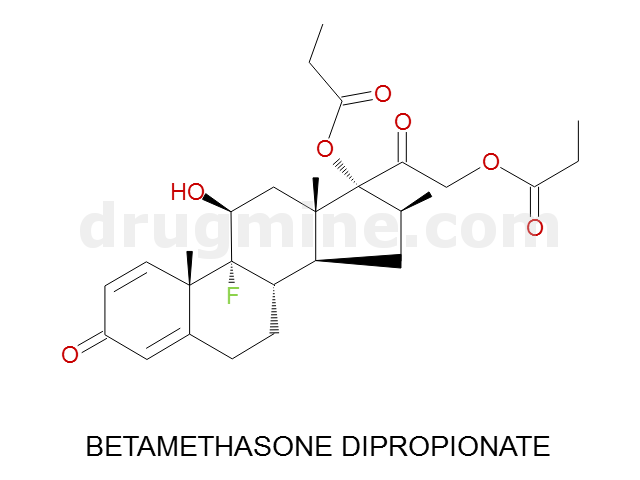

Name: BETAMETHASONE DIPROPIONATE
ID :
MW: 505
Number of atoms: 36
Molecular_Formula: C28H37FO7
Alogp: 3.8
Indication class : Glucocorticoid
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
betamethasone dipropionate containing products summary
There are in total 58 different products containing the active ingredient betamethasone dipropionate. From the 58 drug products, 22 have been discontinued.Product id = 4124
Application Number = 17536
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DIPROSONE
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12451
Application Number = 17691
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DIPROSONE
Route/format = TOPICAL / OINTMENT
Application Type = N
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12191
Application Number = 17781
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DIPROSONE
Route/format = TOPICAL / LOTION
Application Type = N
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 4512
Application Number = 17829
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = DIPROSONE
Route/format = TOPICAL / DISC
Application Type = N
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.1% BASE
----
Product id = 12293
Application Number = 18741
Date of Application = 27,, Jul, 1983
RX/OTC/DISCN = RX
Tradename = DIPROLENE
Route/format = TOPICAL / OINTMENT, AUGMENTED
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 0.05% BASE
----
Product id = 4299
Application Number = 18827
Date of Application = 10,, Jul, 1984
RX/OTC/DISCN = RX
Tradename = LOTRISONE
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 0.05% BASE;1%
----
Product id = 4061
Application Number = 19136
Date of Application = 26,, Jun, 1984
RX/OTC/DISCN = DISCN
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = PHARMADERM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 4058
Application Number = 19137
Date of Application = 26,, Jun, 1984
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 0.05% BASE
----
Product id = 4030
Application Number = 19138
Date of Application = 26,, Jun, 1984
RX/OTC/DISCN = DISCN
Tradename = ALPHATREX
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = SAVAGE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12412
Application Number = 19140
Date of Application = 4,, Sep, 1984
RX/OTC/DISCN = DISCN
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / OINTMENT
Application Type = N
Applicant Name = PHARMADERM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12410
Application Number = 19141
Date of Application = 4,, Sep, 1984
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / OINTMENT
Application Type = N
Applicant Name = FOUGERA
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 0.05% BASE
----
Product id = 12393
Application Number = 19143
Date of Application = 4,, Sep, 1984
RX/OTC/DISCN = DISCN
Tradename = ALPHATREX
Route/format = TOPICAL / OINTMENT
Application Type = N
Applicant Name = SAVAGE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 4006
Application Number = 19408
Date of Application = 31,, Jan, 1986
RX/OTC/DISCN = DISCN
Tradename = DIPROLENE
Route/format = TOPICAL / CREAM, AUGMENTED
Application Type = N
Applicant Name = SCHERING
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 5336
Application Number = 19408
Date of Application = 22,, Nov, 1991
RX/OTC/DISCN = DISCN
Tradename = DIPROLENE
Route/format = TOPICAL / GEL, AUGMENTED
Application Type = N
Applicant Name = SCHERING
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 4007
Application Number = 19555
Date of Application = 27,, Apr, 1987
RX/OTC/DISCN = RX
Tradename = DIPROLENE AF
Route/format = TOPICAL / CREAM, AUGMENTED
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 0.05% BASE
----
Product id = 12135
Application Number = 19716
Date of Application = 1,, Aug, 1988
RX/OTC/DISCN = RX
Tradename = DIPROLENE
Route/format = TOPICAL / LOTION, AUGMENTED
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 0.05% BASE
----
Product id = 12231
Application Number = 20010
Date of Application = 8,, Dec, 2000
RX/OTC/DISCN = RX
Tradename = LOTRISONE
Route/format = TOPICAL / LOTION
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 0.05% BASE;1%
----
Product id = 12576
Application Number = 21852
Date of Application = 9,, Jan, 2006
RX/OTC/DISCN = RX
Tradename = TACLONEX
Route/format = TOPICAL / OINTMENT
Application Type = N
Applicant Name = LEO PHARMA AS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.064%;0.005%
----
Product id = 14854
Application Number = 22185
Date of Application = 9,, May, 2008
RX/OTC/DISCN = RX
Tradename = TACLONEX
Route/format = TOPICAL / SUSPENSION
Application Type = N
Applicant Name = LEO PHARMA AS
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.064%;0.005%
----
Product id = 12146
Application Number = 70273
Date of Application = 12,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = ALPHATREX
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = SAVAGE LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12158
Application Number = 70274
Date of Application = 12,, Aug, 1985
RX/OTC/DISCN = DISCN
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = PHARMADERM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12156
Application Number = 70275
Date of Application = 12,, Aug, 1985
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = FOUGERA
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 0.05% BASE
----
Product id = 12154
Application Number = 70281
Date of Application = 31,, Jul, 1985
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 4057
Application Number = 70885
Date of Application = 3,, Feb, 1987
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12409
Application Number = 71012
Date of Application = 3,, Feb, 1987
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12155
Application Number = 71085
Date of Application = 3,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = ALPHARMA US PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 4062
Application Number = 71143
Date of Application = 17,, Jun, 1987
RX/OTC/DISCN = DISCN
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12161
Application Number = 71467
Date of Application = 10,, Aug, 1987
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 4064
Application Number = 71476
Date of Application = 10,, Aug, 1987
RX/OTC/DISCN = DISCN
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12414
Application Number = 71477
Date of Application = 10,, Aug, 1987
RX/OTC/DISCN = DISCN
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12162
Application Number = 71882
Date of Application = 6,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12159
Application Number = 72276
Date of Application = 24,, Aug, 1988
RX/OTC/DISCN = DISCN
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12411
Application Number = 72526
Date of Application = 31,, Jan, 1990
RX/OTC/DISCN = DISCN
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 4059
Application Number = 72536
Date of Application = 31,, Jan, 1990
RX/OTC/DISCN = DISCN
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12157
Application Number = 72538
Date of Application = 31,, Jan, 1990
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 4063
Application Number = 73552
Date of Application = 30,, Apr, 1992
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12413
Application Number = 74271
Date of Application = 15,, Sep, 1994
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12160
Application Number = 74272
Date of Application = 30,, Sep, 1994
RX/OTC/DISCN = DISCN
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12290
Application Number = 74304
Date of Application = 31,, Aug, 1995
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / OINTMENT, AUGMENTED
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 4060
Application Number = 74579
Date of Application = 26,, Nov, 1997
RX/OTC/DISCN = DISCN
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 5334
Application Number = 75276
Date of Application = 13,, May, 2003
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / GEL, AUGMENTED
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 0.05% BASE
----
Product id = 12291
Application Number = 75373
Date of Application = 22,, Jun, 1999
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / OINTMENT, AUGMENTED
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 4097
Application Number = 75502
Date of Application = 5,, Jun, 2001
RX/OTC/DISCN = RX
Tradename = CLOTRIMAZOLE AND BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE;1%
----
Product id = 4098
Application Number = 75673
Date of Application = 29,, May, 2001
RX/OTC/DISCN = RX
Tradename = CLOTRIMAZOLE AND BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE;1%
----
Product id = 4096
Application Number = 76002
Date of Application = 2,, Aug, 2002
RX/OTC/DISCN = RX
Tradename = CLOTRIMAZOLE AND BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE;1%
----
Product id = 4001
Application Number = 76215
Date of Application = 9,, Dec, 2003
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM, AUGMENTED
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12178
Application Number = 76493
Date of Application = 28,, Jul, 2004
RX/OTC/DISCN = RX
Tradename = CLOTRIMAZOLE AND BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE;1%
----
Product id = 5335
Application Number = 76508
Date of Application = 2,, Dec, 2003
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / GEL, AUGMENTED
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12177
Application Number = 76516
Date of Application = 16,, Jun, 2005
RX/OTC/DISCN = RX
Tradename = CLOTRIMAZOLE AND BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE;1%
----
Product id = 4004
Application Number = 76543
Date of Application = 9,, Dec, 2003
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM, AUGMENTED
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 4003
Application Number = 76592
Date of Application = 9,, Dec, 2003
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM, AUGMENTED
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 4005
Application Number = 76603
Date of Application = 23,, Jan, 2004
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM, AUGMENTED
Application Type = A
Applicant Name = TOLMAR
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12292
Application Number = 76753
Date of Application = 12,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / OINTMENT, AUGMENTED
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12133
Application Number = 77111
Date of Application = 21,, May, 2007
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / LOTION, AUGMENTED
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12134
Application Number = 77477
Date of Application = 21,, May, 2007
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / LOTION, AUGMENTED
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 4002
Application Number = 78930
Date of Application = 23,, Sep, 2008
RX/OTC/DISCN = RX
Tradename = BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / CREAM, AUGMENTED
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 0.05% BASE
----
Product id = 12422
Application Number = 200174
Date of Application = 12,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = CALCIPOTRIENE AND BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = PERRIGO ISRAEL
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.064%;0.005%
----
Product id = 12423
Application Number = 201615
Date of Application = 14,, Jan, 2013
RX/OTC/DISCN = RX
Tradename = CALCIPOTRIENE AND BETAMETHASONE DIPROPIONATE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = TOLMAR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.064%;0.005%
----