bisoprolol-fumarate
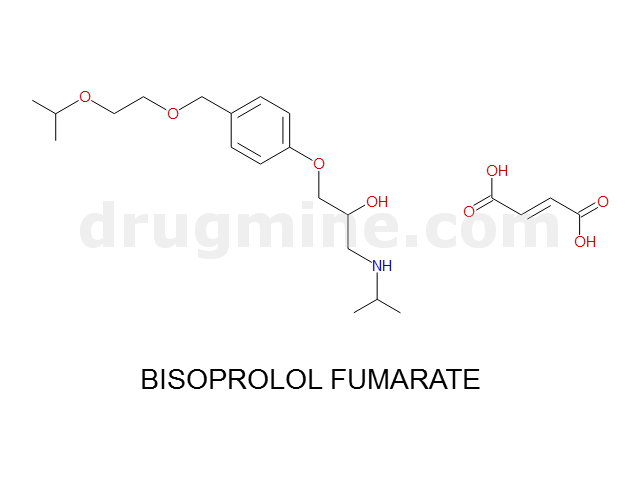
Name: BISOPROLOL FUMARATE
ID :
MW: 325
Number of atoms: 23
Molecular_Formula: C18H31NO4
Alogp: 2.031
Indication class : Antihypertensive (beta-blocker)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
bisoprolol fumarate containing products summary
There are in total 44 different products containing the active ingredient bisoprolol fumarate. From the 44 drug products, 20 have been discontinued.Product id = 29766
Application Number = 19982
Date of Application = 31,, Jul, 1992
RX/OTC/DISCN = RX
Tradename = ZEBETA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TEVA WOMENS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 10MG
----
Product id = 29765
Application Number = 19982
Date of Application = 31,, Jul, 1992
RX/OTC/DISCN = RX
Tradename = ZEBETA
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TEVA WOMENS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 29781
Application Number = 20186
Date of Application = 26,, Mar, 1993
RX/OTC/DISCN = RX
Tradename = ZIAC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TEVA WOMENS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 29782
Application Number = 20186
Date of Application = 26,, Mar, 1993
RX/OTC/DISCN = RX
Tradename = ZIAC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TEVA WOMENS
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 10MG;6.25MG
----
Product id = 29780
Application Number = 20186
Date of Application = 26,, Mar, 1993
RX/OTC/DISCN = RX
Tradename = ZIAC
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = TEVA WOMENS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18602
Application Number = 75469
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18603
Application Number = 75469
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18604
Application Number = 75469
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG;6.25MG
----
Product id = 18568
Application Number = 75474
Date of Application = 25,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 18569
Application Number = 75474
Date of Application = 25,, Oct, 2002
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MUTUAL PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 18590
Application Number = 75527
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18594
Application Number = 75527
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG;6.25MG
----
Product id = 18592
Application Number = 75527
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18591
Application Number = 75579
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18593
Application Number = 75579
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18595
Application Number = 75579
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG;6.25MG
----
Product id = 18584
Application Number = 75632
Date of Application = 27,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18585
Application Number = 75632
Date of Application = 27,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18586
Application Number = 75632
Date of Application = 27,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG;6.25MG
----
Product id = 18582
Application Number = 75642
Date of Application = 27,, Dec, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18581
Application Number = 75642
Date of Application = 27,, Dec, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18583
Application Number = 75642
Date of Application = 27,, Dec, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTHECON
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG;6.25MG
----
Product id = 18572
Application Number = 75643
Date of Application = 16,, Nov, 2000
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18573
Application Number = 75643
Date of Application = 16,, Nov, 2000
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18574
Application Number = 75644
Date of Application = 26,, Jun, 2001
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18575
Application Number = 75644
Date of Application = 26,, Jun, 2001
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18578
Application Number = 75672
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18579
Application Number = 75672
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18580
Application Number = 75672
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACTAVIS ELIZABETH
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG;6.25MG
----
Product id = 18596
Application Number = 75686
Date of Application = 19,, Jan, 2001
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18597
Application Number = 75686
Date of Application = 19,, Jan, 2001
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18598
Application Number = 75686
Date of Application = 19,, Jan, 2001
RX/OTC/DISCN = DISCN
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG;6.25MG
----
Product id = 18587
Application Number = 75768
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18588
Application Number = 75768
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18589
Application Number = 75768
Date of Application = 25,, Sep, 2000
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG;6.25MG
----
Product id = 18570
Application Number = 75831
Date of Application = 14,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18571
Application Number = 75831
Date of Application = 14,, Dec, 2005
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18566
Application Number = 77910
Date of Application = 27,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18567
Application Number = 77910
Date of Application = 27,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18576
Application Number = 78635
Date of Application = 18,, Aug, 2009
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNICHEM PHARMS (USA)
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18577
Application Number = 78635
Date of Application = 18,, Aug, 2009
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNICHEM PHARMS (USA)
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18599
Application Number = 79106
Date of Application = 28,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNICHEM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG;6.25MG
----
Product id = 18600
Application Number = 79106
Date of Application = 28,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNICHEM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG;6.25MG
----
Product id = 18601
Application Number = 79106
Date of Application = 28,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = BISOPROLOL FUMARATE AND HYDROCHLOROTHIAZIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNICHEM
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG;6.25MG
----