buspirone-hydrochloride
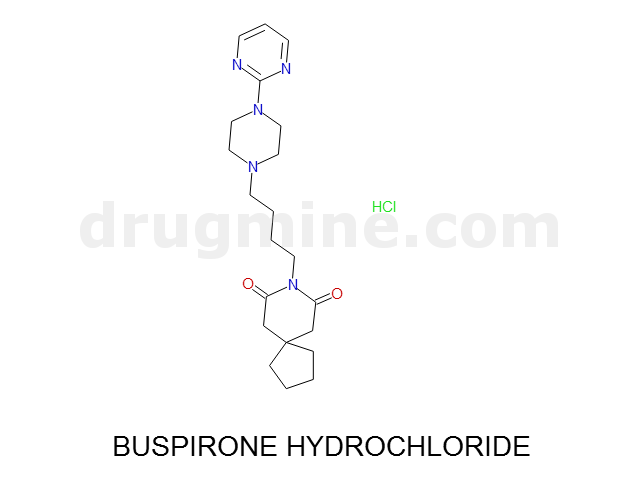


Name: BUSPIRONE HYDROCHLORIDE
ID :
MW: 386
Number of atoms: 28
Molecular_Formula: C21H31N5O2
Alogp: 2.021
Indication class : Tranquilizer (minor)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
buspirone hydrochloride containing products summary
There are in total 59 different products containing the active ingredient buspirone hydrochloride. From the 59 drug products, 26 have been discontinued.Product id = 18658
Application Number = 18731
Date of Application = 29,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = BUSPAR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 18659
Application Number = 18731
Date of Application = 29,, Sep, 1986
RX/OTC/DISCN = DISCN
Tradename = BUSPAR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 18660
Application Number = 18731
Date of Application = 22,, Apr, 1996
RX/OTC/DISCN = DISCN
Tradename = BUSPAR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 003
Tecode =
Rld = No
Strength = 15MG
----
Product id = 18661
Application Number = 18731
Date of Application = 22,, Apr, 1996
RX/OTC/DISCN = DISCN
Tradename = BUSPAR
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 004
Tecode =
Rld = No
Strength = 30MG
----
Product id = 1169
Application Number = 21190
Date of Application = 20,, Dec, 2000
RX/OTC/DISCN = DISCN
Tradename = BUSPAR
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 1170
Application Number = 21190
Date of Application = 20,, Dec, 2000
RX/OTC/DISCN = DISCN
Tradename = BUSPAR
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 002
Tecode =
Rld = No
Strength = 7.5MG
----
Product id = 1171
Application Number = 21190
Date of Application = 20,, Dec, 2000
RX/OTC/DISCN = DISCN
Tradename = BUSPAR
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG
----
Product id = 1172
Application Number = 21190
Date of Application = 20,, Dec, 2000
RX/OTC/DISCN = DISCN
Tradename = BUSPAR
Route/format = ORAL / CAPSULE
Application Type = N
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 004
Tecode =
Rld = No
Strength = 15MG
----
Product id = 18706
Application Number = 74253
Date of Application = 28,, Mar, 2001
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18707
Application Number = 74253
Date of Application = 28,, Mar, 2001
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18708
Application Number = 74253
Date of Application = 13,, Mar, 2002
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = 15MG
----
Product id = 18702
Application Number = 75022
Date of Application = 28,, Feb, 2002
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18703
Application Number = 75022
Date of Application = 28,, Feb, 2002
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18704
Application Number = 75022
Date of Application = 28,, Feb, 2002
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 15MG
----
Product id = 18705
Application Number = 75022
Date of Application = 25,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 004
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 18674
Application Number = 75119
Date of Application = 14,, Mar, 2002
RX/OTC/DISCN = DISCN
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EGIS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 18675
Application Number = 75119
Date of Application = 14,, Mar, 2002
RX/OTC/DISCN = DISCN
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EGIS
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 18676
Application Number = 75119
Date of Application = 23,, Jan, 2003
RX/OTC/DISCN = DISCN
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = EGIS
ProductNo = 003
Tecode =
Rld = No
Strength = 15MG
----
Product id = 18680
Application Number = 75272
Date of Application = 1,, Mar, 2002
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18683
Application Number = 75272
Date of Application = 1,, Mar, 2002
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18685
Application Number = 75272
Date of Application = 28,, Mar, 2001
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 15MG
----
Product id = 18677
Application Number = 75385
Date of Application = 1,, Mar, 2002
RX/OTC/DISCN = DISCN
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 18678
Application Number = 75385
Date of Application = 1,, Mar, 2002
RX/OTC/DISCN = DISCN
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 18679
Application Number = 75385
Date of Application = 1,, Mar, 2002
RX/OTC/DISCN = DISCN
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 15MG
----
Product id = 18691
Application Number = 75388
Date of Application = 9,, May, 2002
RX/OTC/DISCN = DISCN
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 18692
Application Number = 75388
Date of Application = 9,, May, 2002
RX/OTC/DISCN = DISCN
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 18693
Application Number = 75388
Date of Application = 9,, May, 2002
RX/OTC/DISCN = DISCN
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 003
Tecode =
Rld = No
Strength = 15MG
----
Product id = 18695
Application Number = 75413
Date of Application = 19,, Mar, 2002
RX/OTC/DISCN = DISCN
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 18696
Application Number = 75413
Date of Application = 19,, Mar, 2002
RX/OTC/DISCN = DISCN
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 18697
Application Number = 75413
Date of Application = 19,, Mar, 2002
RX/OTC/DISCN = DISCN
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = 15MG
----
Product id = 18681
Application Number = 75467
Date of Application = 28,, Feb, 2002
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18682
Application Number = 75467
Date of Application = 28,, Mar, 2001
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = 7.5MG
----
Product id = 18684
Application Number = 75467
Date of Application = 28,, Feb, 2002
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18686
Application Number = 75467
Date of Application = 28,, Feb, 2002
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = 15MG
----
Product id = 18667
Application Number = 75521
Date of Application = 5,, Apr, 2002
RX/OTC/DISCN = DISCN
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 18668
Application Number = 75521
Date of Application = 5,, Apr, 2002
RX/OTC/DISCN = DISCN
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 18669
Application Number = 75521
Date of Application = 5,, Apr, 2002
RX/OTC/DISCN = DISCN
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 003
Tecode =
Rld = No
Strength = 15MG
----
Product id = 18688
Application Number = 75572
Date of Application = 27,, Feb, 2002
RX/OTC/DISCN = DISCN
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NESHER PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 5MG
----
Product id = 18689
Application Number = 75572
Date of Application = 27,, Feb, 2002
RX/OTC/DISCN = DISCN
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NESHER PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = 10MG
----
Product id = 18690
Application Number = 75572
Date of Application = 27,, Feb, 2002
RX/OTC/DISCN = DISCN
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NESHER PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = 15MG
----
Product id = 18687
Application Number = 76008
Date of Application = 28,, Jun, 2001
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 18670
Application Number = 78246
Date of Application = 27,, Feb, 2009
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18671
Application Number = 78246
Date of Application = 27,, Feb, 2009
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18672
Application Number = 78246
Date of Application = 27,, Feb, 2009
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 15MG
----
Product id = 18673
Application Number = 78246
Date of Application = 27,, Feb, 2009
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 18694
Application Number = 78302
Date of Application = 17,, Dec, 2007
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PROSAM LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 18709
Application Number = 78888
Date of Application = 7,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18710
Application Number = 78888
Date of Application = 7,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18711
Application Number = 78888
Date of Application = 7,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 15MG
----
Product id = 18712
Application Number = 78888
Date of Application = 7,, Feb, 2014
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ZYDUS PHARMS USA INC
ProductNo = 004
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 18698
Application Number = 202330
Date of Application = 25,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = STRIDES ARCOLAB LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18699
Application Number = 202330
Date of Application = 25,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = STRIDES ARCOLAB LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18700
Application Number = 202330
Date of Application = 25,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = STRIDES ARCOLAB LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = 15MG
----
Product id = 18701
Application Number = 202330
Date of Application = 25,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = STRIDES ARCOLAB LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = 30MG
----
Product id = 18662
Application Number = 202557
Date of Application = 30,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 18663
Application Number = 202557
Date of Application = 30,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 002
Tecode = AB
Rld = No
Strength = 7.5MG
----
Product id = 18664
Application Number = 202557
Date of Application = 30,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 003
Tecode = AB
Rld = No
Strength = 10MG
----
Product id = 18665
Application Number = 202557
Date of Application = 30,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 004
Tecode = AB
Rld = No
Strength = 15MG
----
Product id = 18666
Application Number = 202557
Date of Application = 30,, Dec, 2014
RX/OTC/DISCN = RX
Tradename = BUSPIRONE HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ACCORD HLTHCARE
ProductNo = 005
Tecode = AB
Rld = No
Strength = 30MG
----