carbenicillin-disodium
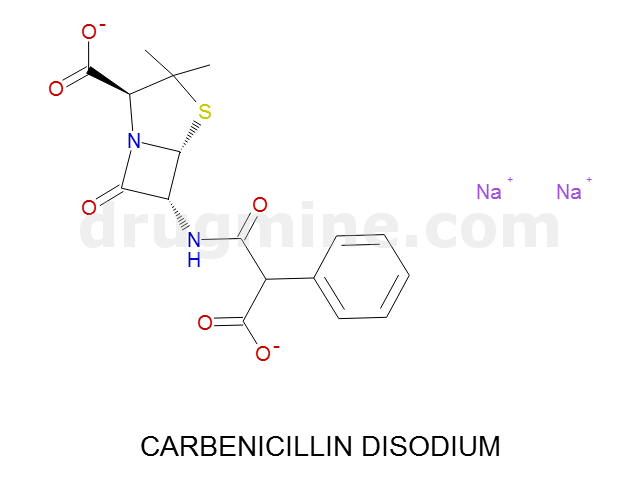


Name: CARBENICILLIN DISODIUM
ID :
MW: 376
Number of atoms: 26
Molecular_Formula: C17H16N2O6S
Alogp: -2.218
Indication class : Antibacterial
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
carbenicillin disodium containing products summary
There are in total 10 different products containing the active ingredient carbenicillin disodium. From the 10 drug products, 10 have been discontinued.Product id = 10584
Application Number = 50298
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PYOPEN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 10585
Application Number = 50298
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PYOPEN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 10586
Application Number = 50298
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PYOPEN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 5GM BASE/VIAL
----
Product id = 10587
Application Number = 50298
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PYOPEN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 006
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 10588
Application Number = 50298
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = PYOPEN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 007
Tecode =
Rld = No
Strength = EQ 20GM BASE/VIAL
----
Product id = 7941
Application Number = 50306
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = GEOPEN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ROERIG
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 7943
Application Number = 50306
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = GEOPEN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ROERIG
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 5GM BASE/VIAL
----
Product id = 7942
Application Number = 50306
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = GEOPEN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ROERIG
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 7944
Application Number = 50306
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = GEOPEN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ROERIG
ProductNo = 006
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 7945
Application Number = 50306
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = GEOPEN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ROERIG
ProductNo = 007
Tecode =
Rld = No
Strength = EQ 30GM BASE/VIAL
----