carteolol-hydrochloride
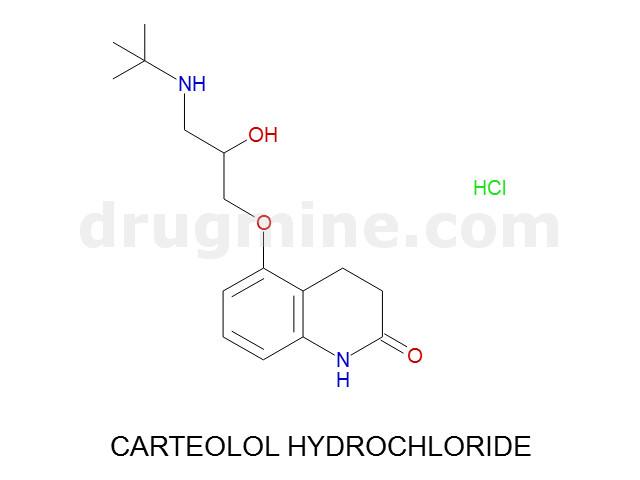


Name: CARTEOLOL HYDROCHLORIDE
ID :
MW: 292
Number of atoms: 21
Molecular_Formula: C16H24N2O3
Alogp: 1.282
Indication class : Anti-Adrenergic (beta-receptor)
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
carteolol hydrochloride containing products summary
There are in total 7 different products containing the active ingredient carteolol hydrochloride. From the 7 drug products, 4 have been discontinued.Product id = 19042
Application Number = 19204
Date of Application = 28,, Dec, 1988
RX/OTC/DISCN = DISCN
Tradename = CARTROL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBVIE
ProductNo = 001
Tecode =
Rld = No
Strength = 2.5MG
----
Product id = 19043
Application Number = 19204
Date of Application = 28,, Dec, 1988
RX/OTC/DISCN = DISCN
Tradename = CARTROL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBVIE
ProductNo = 002
Tecode =
Rld = No
Strength = 5MG
----
Product id = 19044
Application Number = 19204
Date of Application = 28,, Dec, 1988
RX/OTC/DISCN = DISCN
Tradename = CARTROL
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = ABBVIE
ProductNo = 003
Tecode =
Rld = No
Strength = 10MG
----
Product id = 13057
Application Number = 19972
Date of Application = 23,, May, 1990
RX/OTC/DISCN = RX
Tradename = OCUPRESS
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = NOVARTIS
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 1%
----
Product id = 12894
Application Number = 75476
Date of Application = 3,, Jan, 2000
RX/OTC/DISCN = RX
Tradename = CARTEOLOL HYDROCHLORIDE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = ALCON
ProductNo = 001
Tecode = AT
Rld = No
Strength = 1%
----
Product id = 12896
Application Number = 75546
Date of Application = 20,, Jan, 2000
RX/OTC/DISCN = RX
Tradename = CARTEOLOL HYDROCHLORIDE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = BAUSCH AND LOMB
ProductNo = 001
Tecode = AT
Rld = No
Strength = 1%
----
Product id = 12895
Application Number = 76097
Date of Application = 6,, Feb, 2002
RX/OTC/DISCN = DISCN
Tradename = CARTEOLOL HYDROCHLORIDE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 1%
----