cefazolin-sodium

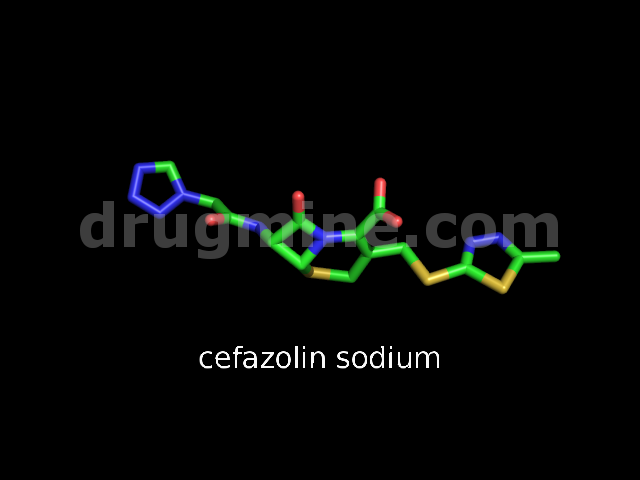
Name: CEFAZOLIN SODIUM
ID :
MW: 453
Number of atoms: 29
Molecular_Formula: C14H13N8O4S3
Alogp: -2.807
Indication class : Antibacterial (systemic)
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
cefazolin sodium containing products summary
There are in total 81 different products containing the active ingredient cefazolin sodium. From the 81 drug products, 53 have been discontinued.Product id = 5970
Application Number = 50461
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ANCEF
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/VIAL **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 5971
Application Number = 50461
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ANCEF
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 5967
Application Number = 50461
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ANCEF
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 5968
Application Number = 50461
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ANCEF
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 5GM BASE/VIAL **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 5969
Application Number = 50461
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = ANCEF
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = GLAXOSMITHKLINE
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 5976
Application Number = 50566
Date of Application = 8,, Jun, 1983
RX/OTC/DISCN = DISCN
Tradename = ANCEF IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE/ML
----
Product id = 5977
Application Number = 50566
Date of Application = 8,, Jun, 1983
RX/OTC/DISCN = DISCN
Tradename = ANCEF IN SODIUM CHLORIDE 0.9% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20MG BASE/ML
----
Product id = 5972
Application Number = 50566
Date of Application = 8,, Jun, 1983
RX/OTC/DISCN = DISCN
Tradename = ANCEF IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 10MG BASE/ML
----
Product id = 5973
Application Number = 50566
Date of Application = 8,, Jun, 1983
RX/OTC/DISCN = DISCN
Tradename = ANCEF IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 20MG BASE/ML
----
Product id = 6335
Application Number = 50779
Date of Application = 27,, Jul, 2000
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN AND DEXTROSE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6333
Application Number = 50779
Date of Application = 27,, Jul, 2000
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN AND DEXTROSE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 1GM BASE/VIAL
----
Product id = 6334
Application Number = 50779
Date of Application = 13,, Jan, 2012
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN AND DEXTROSE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 8601
Application Number = 61773
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = KEFZOL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 8602
Application Number = 61773
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = KEFZOL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 8598
Application Number = 61773
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = KEFZOL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 8599
Application Number = 61773
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = KEFZOL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR
ProductNo = 004
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 8600
Application Number = 61773
Date of Application = 8,, Sep, 1987
RX/OTC/DISCN = DISCN
Tradename = KEFZOL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 20GM BASE/VIAL
----
Product id = 8604
Application Number = 62557
Date of Application = 10,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = KEFZOL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LILLY
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 8603
Application Number = 62557
Date of Application = 10,, Sep, 1985
RX/OTC/DISCN = DISCN
Tradename = KEFZOL
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LILLY
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6339
Application Number = 62688
Date of Application = 17,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6336
Application Number = 62688
Date of Application = 17,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6337
Application Number = 62688
Date of Application = 17,, Nov, 1986
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6338
Application Number = 62688
Date of Application = 3,, Aug, 1987
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 20GM BASE/VIAL
----
Product id = 6371
Application Number = 62807
Date of Application = 12,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 6372
Application Number = 62807
Date of Application = 12,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6367
Application Number = 62807
Date of Application = 12,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6368
Application Number = 62807
Date of Application = 12,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 5GM BASE/VIAL
----
Product id = 6369
Application Number = 62807
Date of Application = 12,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6370
Application Number = 62807
Date of Application = 12,, Jan, 1988
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 006
Tecode =
Rld = No
Strength = EQ 20GM BASE/VIAL
----
Product id = 6382
Application Number = 62831
Date of Application = 9,, Dec, 1988
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6379
Application Number = 62831
Date of Application = 9,, Dec, 1988
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6381
Application Number = 62831
Date of Application = 25,, Sep, 1992
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6349
Application Number = 62894
Date of Application = 21,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 6350
Application Number = 62894
Date of Application = 21,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6346
Application Number = 62894
Date of Application = 21,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6347
Application Number = 62894
Date of Application = 21,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 5GM BASE/VIAL
----
Product id = 6348
Application Number = 62894
Date of Application = 21,, Jul, 1988
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6394
Application Number = 62988
Date of Application = 29,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 6395
Application Number = 62988
Date of Application = 29,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6390
Application Number = 62988
Date of Application = 29,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6391
Application Number = 62989
Date of Application = 29,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5GM BASE/VIAL
----
Product id = 6392
Application Number = 62989
Date of Application = 29,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6393
Application Number = 62989
Date of Application = 29,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 20GM BASE/VIAL
----
Product id = 5974
Application Number = 63002
Date of Application = 28,, Mar, 1991
RX/OTC/DISCN = RX
Tradename = ANCEF IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 10MG BASE/ML
----
Product id = 5975
Application Number = 63002
Date of Application = 28,, Mar, 1991
RX/OTC/DISCN = RX
Tradename = ANCEF IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 20MG BASE/ML
----
Product id = 6388
Application Number = 63016
Date of Application = 14,, Mar, 1989
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 6389
Application Number = 63016
Date of Application = 14,, Mar, 1989
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6385
Application Number = 63016
Date of Application = 14,, Mar, 1989
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6386
Application Number = 63018
Date of Application = 5,, Mar, 1990
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 5GM BASE/VIAL
----
Product id = 6387
Application Number = 63018
Date of Application = 5,, Mar, 1990
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6355
Application Number = 63207
Date of Application = 27,, Dec, 1991
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FACTA FARMA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6383
Application Number = 63208
Date of Application = 27,, Dec, 1991
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = STERI PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6356
Application Number = 63209
Date of Application = 27,, Dec, 1991
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FACTA FARMA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6357
Application Number = 63209
Date of Application = 30,, Apr, 1999
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FACTA FARMA
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 20GM BASE/VIAL
----
Product id = 6358
Application Number = 63214
Date of Application = 27,, Dec, 1991
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FACTA FARMA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6384
Application Number = 63216
Date of Application = 27,, Dec, 1991
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = STERI PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6363
Application Number = 64033
Date of Application = 31,, Oct, 1993
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6362
Application Number = 64169
Date of Application = 14,, Aug, 1998
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6359
Application Number = 64169
Date of Application = 14,, Aug, 1998
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6360
Application Number = 64170
Date of Application = 18,, Mar, 1998
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6361
Application Number = 64170
Date of Application = 18,, Mar, 1998
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20GM BASE/VIAL
----
Product id = 6366
Application Number = 65047
Date of Application = 18,, Sep, 2001
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6364
Application Number = 65047
Date of Application = 18,, Sep, 2001
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6377
Application Number = 65141
Date of Application = 29,, Nov, 2006
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAMSON MEDCL
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 100GM BASE/VIAL
----
Product id = 6378
Application Number = 65141
Date of Application = 29,, Nov, 2006
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAMSON MEDCL
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 300GM BASE/VIAL
----
Product id = 6365
Application Number = 65143
Date of Application = 18,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6376
Application Number = 65226
Date of Application = 21,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 500MG BASE/VIAL
----
Product id = 6373
Application Number = 65226
Date of Application = 21,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 1GM BASE/VIAL
----
Product id = 6374
Application Number = 65244
Date of Application = 12,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 1GM BASE/VIAL
----
Product id = 6375
Application Number = 65247
Date of Application = 12,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 10GM BASE/VIAL
----
Product id = 6354
Application Number = 65280
Date of Application = 18,, Mar, 2009
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CEPHAZONE PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6351
Application Number = 65280
Date of Application = 18,, Mar, 2009
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CEPHAZONE PHARMA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6352
Application Number = 65295
Date of Application = 18,, Mar, 2009
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CEPHAZONE PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6353
Application Number = 65296
Date of Application = 18,, Mar, 2009
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = CEPHAZONE PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 20GM BASE/VIAL
----
Product id = 6343
Application Number = 65303
Date of Application = 22,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6340
Application Number = 65303
Date of Application = 22,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6341
Application Number = 65306
Date of Application = 22,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6342
Application Number = 65306
Date of Application = 18,, Aug, 2014
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 20GM BASE/VIAL
----
Product id = 6380
Application Number = 65345
Date of Application = 9,, May, 2007
RX/OTC/DISCN = RX
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6345
Application Number = 65395
Date of Application = 8,, Aug, 2008
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6344
Application Number = 65395
Date of Application = 8,, Aug, 2008
RX/OTC/DISCN = DISCN
Tradename = CEFAZOLIN SODIUM
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----