ceftriaxone-sodium
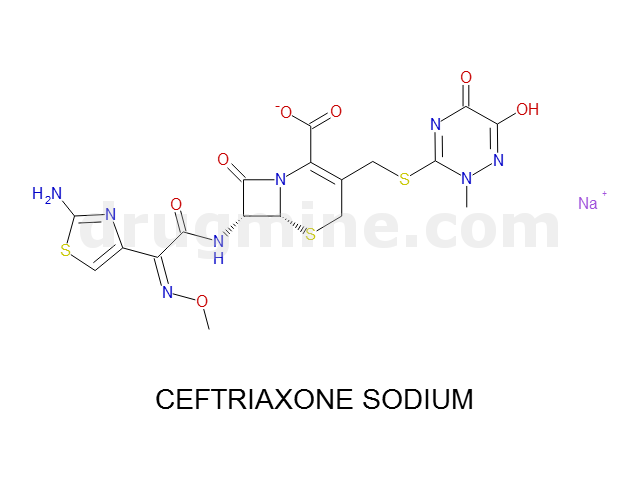


Name: CEFTRIAXONE SODIUM
ID :
MW: 554
Number of atoms: 36
Molecular_Formula: C18H17N8O7S3
Alogp: -1.747
Indication class : Antibacterial
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
ceftriaxone sodium containing products summary
There are in total 91 different products containing the active ingredient ceftriaxone sodium. From the 91 drug products, 40 have been discontinued.Product id = 11587
Application Number = 50585
Date of Application = 21,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = ROCEPHIN
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = N
Applicant Name = HOFFMANN LA ROCHE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 11588
Application Number = 50585
Date of Application = 21,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = ROCEPHIN
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = N
Applicant Name = HOFFMANN LA ROCHE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 11585
Application Number = 50585
Date of Application = 21,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = ROCEPHIN
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = N
Applicant Name = HOFFMANN LA ROCHE
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 11586
Application Number = 50585
Date of Application = 21,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = ROCEPHIN
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = N
Applicant Name = HOFFMANN LA ROCHE
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 10673
Application Number = 50585
Date of Application = 21,, Dec, 1984
RX/OTC/DISCN = DISCN
Tradename = ROCEPHIN
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOFFMANN LA ROCHE
ProductNo = 005
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 10680
Application Number = 50585
Date of Application = 8,, May, 1996
RX/OTC/DISCN = DISCN
Tradename = ROCEPHIN KIT
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOFFMANN LA ROCHE
ProductNo = 006
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL,N/A;N/A,1%
----
Product id = 10681
Application Number = 50585
Date of Application = 8,, May, 1996
RX/OTC/DISCN = DISCN
Tradename = ROCEPHIN KIT
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOFFMANN LA ROCHE
ProductNo = 007
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL,N/A;N/A,1%
----
Product id = 10682
Application Number = 50624
Date of Application = 11,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = ROCEPHIN W/ DEXTROSE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOFFMANN LA ROCHE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10MG BASE/ML
----
Product id = 10683
Application Number = 50624
Date of Application = 11,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = ROCEPHIN W/ DEXTROSE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOFFMANN LA ROCHE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 20MG BASE/ML
----
Product id = 10684
Application Number = 50624
Date of Application = 11,, Feb, 1987
RX/OTC/DISCN = DISCN
Tradename = ROCEPHIN W/ DEXTROSE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = HOFFMANN LA ROCHE
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 40MG BASE/ML
----
Product id = 6531
Application Number = 50796
Date of Application = 20,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE AND DEXTROSE IN DUPLEX CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 1GM BASE/VIAL
----
Product id = 6532
Application Number = 50796
Date of Application = 20,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE AND DEXTROSE IN DUPLEX CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = B BRAUN
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 2GM BASE/VIAL
----
Product id = 10678
Application Number = 62510
Date of Application = 12,, Mar, 1985
RX/OTC/DISCN = DISCN
Tradename = ROCEPHIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ROCHE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 10679
Application Number = 62510
Date of Application = 12,, Mar, 1985
RX/OTC/DISCN = DISCN
Tradename = ROCEPHIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ROCHE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 10677
Application Number = 62510
Date of Application = 12,, Mar, 1985
RX/OTC/DISCN = DISCN
Tradename = ROCEPHIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ROCHE
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 10675
Application Number = 62654
Date of Application = 30,, Apr, 1987
RX/OTC/DISCN = DISCN
Tradename = ROCEPHIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOFFMANN LA ROCHE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 10670
Application Number = 62654
Date of Application = 30,, Apr, 1987
RX/OTC/DISCN = DISCN
Tradename = ROCEPHIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOFFMANN LA ROCHE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 10672
Application Number = 62654
Date of Application = 30,, Apr, 1987
RX/OTC/DISCN = DISCN
Tradename = ROCEPHIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOFFMANN LA ROCHE
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 10674
Application Number = 63239
Date of Application = 13,, Aug, 1993
RX/OTC/DISCN = DISCN
Tradename = ROCEPHIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOFFMANN LA ROCHE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 10676
Application Number = 63239
Date of Application = 13,, Aug, 1993
RX/OTC/DISCN = RX
Tradename = ROCEPHIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOFFMANN LA ROCHE
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 500MG BASE/VIAL
----
Product id = 10671
Application Number = 63239
Date of Application = 13,, Aug, 1993
RX/OTC/DISCN = RX
Tradename = ROCEPHIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOFFMANN LA ROCHE
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = EQ 1GM BASE/VIAL
----
Product id = 11560
Application Number = 65125
Date of Application = 30,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = LUPIN
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 11561
Application Number = 65125
Date of Application = 30,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = LUPIN
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 11558
Application Number = 65125
Date of Application = 30,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = LUPIN
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 11559
Application Number = 65125
Date of Application = 30,, Sep, 2003
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = LUPIN
ProductNo = 004
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6526
Application Number = 65168
Date of Application = 17,, May, 2005
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 10GM BASE/VIAL
----
Product id = 11564
Application Number = 65169
Date of Application = 9,, May, 2005
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 250MG BASE/VIAL
----
Product id = 11565
Application Number = 65169
Date of Application = 9,, May, 2005
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 500MG BASE/VIAL
----
Product id = 11562
Application Number = 65169
Date of Application = 9,, May, 2005
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = EQ 1GM BASE/VIAL
----
Product id = 11563
Application Number = 65169
Date of Application = 9,, May, 2005
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode = AP
Rld = Yes
Strength = EQ 2GM BASE/VIAL
----
Product id = 6530
Application Number = 65180
Date of Application = 12,, May, 2006
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6527
Application Number = 65204
Date of Application = 3,, May, 2005
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 1GM BASE/VIAL
----
Product id = 6528
Application Number = 65204
Date of Application = 3,, May, 2005
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 2GM BASE/VIAL
----
Product id = 6533
Application Number = 65224
Date of Application = 23,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 20MG BASE/ML
----
Product id = 6534
Application Number = 65224
Date of Application = 23,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode =
Rld = Yes
Strength = EQ 40MG BASE/ML
----
Product id = 11570
Application Number = 65227
Date of Application = 15,, Mar, 2007
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 11571
Application Number = 65227
Date of Application = 15,, Mar, 2007
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 11568
Application Number = 65227
Date of Application = 15,, Mar, 2007
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 11569
Application Number = 65227
Date of Application = 15,, Mar, 2007
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 11556
Application Number = 65230
Date of Application = 2,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 11557
Application Number = 65230
Date of Application = 2,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 11554
Application Number = 65230
Date of Application = 2,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 11555
Application Number = 65230
Date of Application = 2,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 004
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6519
Application Number = 65231
Date of Application = 2,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6521
Application Number = 65231
Date of Application = 2,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6523
Application Number = 65232
Date of Application = 2,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 11548
Application Number = 65245
Date of Application = 15,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 11549
Application Number = 65245
Date of Application = 15,, Feb, 2006
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 11546
Application Number = 65245
Date of Application = 15,, Feb, 2006
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 11547
Application Number = 65245
Date of Application = 15,, Feb, 2006
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6518
Application Number = 65252
Date of Application = 15,, Feb, 2006
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 11566
Application Number = 65262
Date of Application = 29,, Jun, 2006
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 11567
Application Number = 65262
Date of Application = 29,, Jun, 2006
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6524
Application Number = 65263
Date of Application = 12,, Sep, 2006
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LUPIN
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 11544
Application Number = 65268
Date of Application = 28,, Feb, 2007
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = FACTA FARMA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 11545
Application Number = 65268
Date of Application = 28,, Feb, 2007
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = FACTA FARMA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6517
Application Number = 65269
Date of Application = 28,, Feb, 2007
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FACTA FARMA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6529
Application Number = 65274
Date of Application = 1,, May, 2006
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 11542
Application Number = 65294
Date of Application = 26,, Mar, 2007
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = CEPHAZONE PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 11543
Application Number = 65294
Date of Application = 26,, Mar, 2007
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = CEPHAZONE PHARMA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 11540
Application Number = 65294
Date of Application = 26,, Mar, 2007
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = CEPHAZONE PHARMA
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 11541
Application Number = 65294
Date of Application = 26,, Mar, 2007
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = CEPHAZONE PHARMA
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 11530
Application Number = 65305
Date of Application = 11,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = AKORN INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 11531
Application Number = 65305
Date of Application = 11,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = AKORN INC
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 11528
Application Number = 65305
Date of Application = 11,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = AKORN INC
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 11529
Application Number = 65305
Date of Application = 11,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = AKORN INC
ProductNo = 004
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6512
Application Number = 65328
Date of Application = 24,, Jul, 2008
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6513
Application Number = 65329
Date of Application = 24,, Jul, 2008
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 6510
Application Number = 65329
Date of Application = 24,, Jul, 2008
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6511
Application Number = 65329
Date of Application = 24,, Jul, 2008
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ACS DOBFAR
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 11552
Application Number = 65342
Date of Application = 10,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 11553
Application Number = 65342
Date of Application = 10,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 11550
Application Number = 65342
Date of Application = 10,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 11551
Application Number = 65342
Date of Application = 10,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = HIKMA FARMACEUTICA
ProductNo = 004
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 11573
Application Number = 65391
Date of Application = 12,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 11574
Application Number = 65391
Date of Application = 12,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 11572
Application Number = 65391
Date of Application = 12,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 11538
Application Number = 65465
Date of Application = 18,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 11539
Application Number = 65465
Date of Application = 18,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 11536
Application Number = 65465
Date of Application = 18,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 11537
Application Number = 65465
Date of Application = 18,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 004
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6516
Application Number = 65475
Date of Application = 18,, Aug, 2008
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6515
Application Number = 65504
Date of Application = 31,, Jul, 2008
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 11534
Application Number = 65505
Date of Application = 31,, Jul, 2008
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/VIAL
----
Product id = 11535
Application Number = 65505
Date of Application = 31,, Jul, 2008
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL
----
Product id = 11532
Application Number = 65505
Date of Application = 31,, Jul, 2008
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 11533
Application Number = 65505
Date of Application = 31,, Jul, 2008
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INTRAMUSCULAR, INTRAVENOUS / INJECTABLE
Application Type = A
Applicant Name = AUROBINDO PHARMA LTD
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 2GM BASE/VIAL
----
Product id = 6525
Application Number = 90057
Date of Application = 25,, Apr, 2014
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAMSON MEDCL
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 100GM BASE/VIAL
----
Product id = 6514
Application Number = 91068
Date of Application = 7,, Jan, 2013
RX/OTC/DISCN = DISCN
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AGILA SPECLTS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 10GM BASE/VIAL
----
Product id = 6520
Application Number = 202563
Date of Application = 20,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 1GM BASE/VIAL
----
Product id = 6522
Application Number = 202563
Date of Application = 20,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = CEFTRIAXONE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 2GM BASE/VIAL
----