ciclopirox
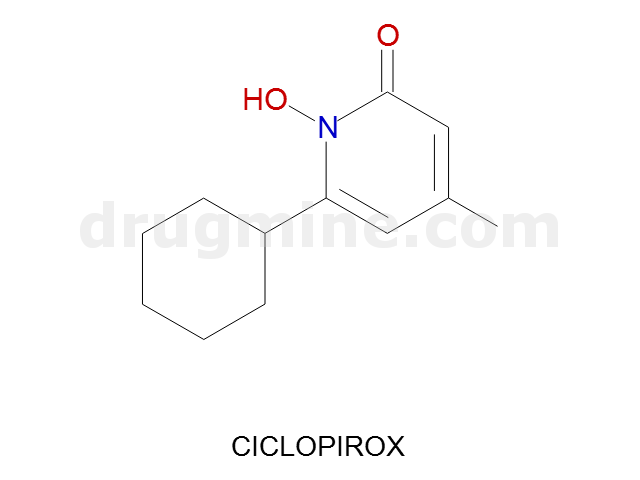


Name: CICLOPIROX
ID :
MW: 207
Number of atoms: 15
Molecular_Formula: C12H17NO2
Alogp: 2.489
Indication class : Antifungal
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
ciclopirox containing products summary
There are in total 31 different products containing the active ingredient ciclopirox. From the 31 drug products, 3 have been discontinued.Product id = 4296
Application Number = 18748
Date of Application = 30,, Dec, 1982
RX/OTC/DISCN = RX
Tradename = LOPROX
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = MEDIMETRIKS PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.77%
----
Product id = 14852
Application Number = 19824
Date of Application = 30,, Dec, 1988
RX/OTC/DISCN = RX
Tradename = LOPROX
Route/format = TOPICAL / SUSPENSION
Application Type = N
Applicant Name = MEDIMETRIKS PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.77%
----
Product id = 5411
Application Number = 20519
Date of Application = 21,, Jul, 1997
RX/OTC/DISCN = RX
Tradename = LOPROX
Route/format = TOPICAL / GEL
Application Type = N
Applicant Name = CNTY LINE PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.77%
----
Product id = 14263
Application Number = 21022
Date of Application = 17,, Dec, 1999
RX/OTC/DISCN = RX
Tradename = PENLAC
Route/format = TOPICAL / SOLUTION
Application Type = N
Applicant Name = VALEANT BERMUDA
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 8%
----
Product id = 12825
Application Number = 21159
Date of Application = 28,, Feb, 2003
RX/OTC/DISCN = RX
Tradename = LOPROX
Route/format = TOPICAL / SHAMPOO
Application Type = N
Applicant Name = MEDICIS
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 1%
----
Product id = 14849
Application Number = 76422
Date of Application = 6,, Aug, 2004
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / SUSPENSION
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.77%
----
Product id = 4079
Application Number = 76435
Date of Application = 29,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.77%
----
Product id = 4083
Application Number = 76790
Date of Application = 12,, Apr, 2005
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.77%
----
Product id = 14851
Application Number = 77092
Date of Application = 10,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / SUSPENSION
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.77%
----
Product id = 4082
Application Number = 77364
Date of Application = 3,, Mar, 2006
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.77%
----
Product id = 14131
Application Number = 77623
Date of Application = 18,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode = AT
Rld = No
Strength = 8%
----
Product id = 14850
Application Number = 77676
Date of Application = 15,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / SUSPENSION
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.77%
----
Product id = 14134
Application Number = 77687
Date of Application = 18,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = TOLMAR
ProductNo = 001
Tecode = AT
Rld = No
Strength = 8%
----
Product id = 5374
Application Number = 77896
Date of Application = 10,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / GEL
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.77%
----
Product id = 14125
Application Number = 78046
Date of Application = 18,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode = AT
Rld = No
Strength = 8%
----
Product id = 14133
Application Number = 78079
Date of Application = 18,, Sep, 2007
RX/OTC/DISCN = DISCN
Tradename = CICLOPIROX
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = TEVA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 8%
----
Product id = 14127
Application Number = 78124
Date of Application = 18,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = CIPLA LTD
ProductNo = 001
Tecode = AT
Rld = No
Strength = 8%
----
Product id = 14132
Application Number = 78144
Date of Application = 18,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = TARO PHARM INDS
ProductNo = 001
Tecode = AT
Rld = No
Strength = 8%
----
Product id = 14126
Application Number = 78172
Date of Application = 18,, Sep, 2007
RX/OTC/DISCN = DISCN
Tradename = CICLOPIROX
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = 8%
----
Product id = 14128
Application Number = 78233
Date of Application = 18,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = G AND W LABS
ProductNo = 001
Tecode = AT
Rld = No
Strength = 8%
----
Product id = 5376
Application Number = 78266
Date of Application = 7,, Jan, 2009
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / GEL
Application Type = A
Applicant Name = PADDOCK LLC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.77%
----
Product id = 14129
Application Number = 78270
Date of Application = 18,, Sep, 2007
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = HI TECH PHARMA
ProductNo = 001
Tecode = AT
Rld = No
Strength = 8%
----
Product id = 4080
Application Number = 78463
Date of Application = 20,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = G AND W LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.77%
----
Product id = 14130
Application Number = 78567
Date of Application = 18,, Sep, 2007
RX/OTC/DISCN = DISCN
Tradename = CICLOPIROX
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 8%
----
Product id = 12813
Application Number = 78594
Date of Application = 16,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / SHAMPOO
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode = AT
Rld = No
Strength = 1%
----
Product id = 14135
Application Number = 78975
Date of Application = 17,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = VERSAPHARM
ProductNo = 001
Tecode = AT
Rld = No
Strength = 8%
----
Product id = 12812
Application Number = 90146
Date of Application = 25,, May, 2010
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / SHAMPOO
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AT
Rld = No
Strength = 1%
----
Product id = 12814
Application Number = 90269
Date of Application = 23,, Feb, 2011
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / SHAMPOO
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AT
Rld = No
Strength = 1%
----
Product id = 4081
Application Number = 90273
Date of Application = 10,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = GLENMARK PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.77%
----
Product id = 12811
Application Number = 90490
Date of Application = 24,, Nov, 2009
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / SHAMPOO
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode = AT
Rld = No
Strength = 1%
----
Product id = 5375
Application Number = 91595
Date of Application = 29,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = CICLOPIROX
Route/format = TOPICAL / GEL
Application Type = A
Applicant Name = GLENMARK GENERICS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.77%
----