cilastatin-sodium
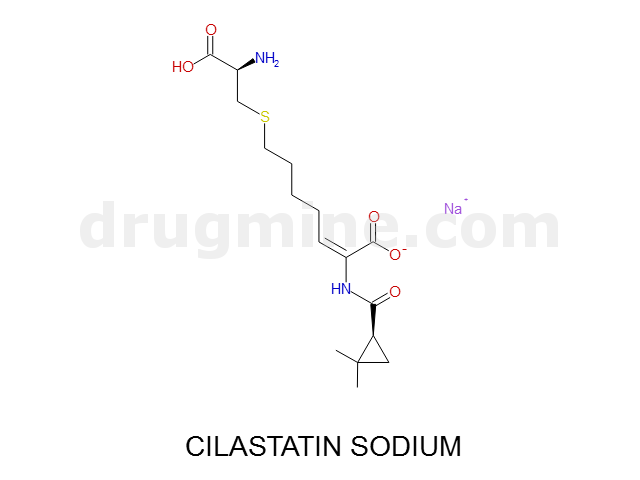


Name: CILASTATIN SODIUM
ID :
MW: 357
Number of atoms: 24
Molecular_Formula: C16H25N2O5S
Alogp: -2.933
Indication class : Enzyme Inhibitor
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
cilastatin sodium containing products summary
There are in total 11 different products containing the active ingredient cilastatin sodium. From the 11 drug products, 3 have been discontinued.Product id = 12716
Application Number = 50587
Date of Application = 26,, Nov, 1985
RX/OTC/DISCN = RX
Tradename = PRIMAXIN
Route/format = INTRAVENOUS / POWDER
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 250MG BASE/VIAL;250MG/VIAL
----
Product id = 12717
Application Number = 50587
Date of Application = 26,, Nov, 1985
RX/OTC/DISCN = RX
Tradename = PRIMAXIN
Route/format = INTRAVENOUS / POWDER
Application Type = N
Applicant Name = MERCK
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 500MG BASE/VIAL;500MG/VIAL
----
Product id = 12694
Application Number = 50630
Date of Application = 14,, Dec, 1990
RX/OTC/DISCN = RX
Tradename = PRIMAXIN
Route/format = INTRAMUSCULAR / POWDER
Application Type = N
Applicant Name = MERCK
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL;500MG/VIAL
----
Product id = 12695
Application Number = 50630
Date of Application = 14,, Dec, 1990
RX/OTC/DISCN = DISCN
Tradename = PRIMAXIN
Route/format = INTRAMUSCULAR / POWDER
Application Type = N
Applicant Name = MERCK
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 750MG BASE/VIAL;750MG/VIAL
----
Product id = 10436
Application Number = 62756
Date of Application = 8,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = PRIMAXIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MERCK
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE/VIAL;250MG/VIAL
----
Product id = 10437
Application Number = 62756
Date of Application = 8,, Jan, 1987
RX/OTC/DISCN = DISCN
Tradename = PRIMAXIN
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MERCK
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE/VIAL;500MG/VIAL
----
Product id = 12705
Application Number = 90577
Date of Application = 21,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = IMIPENEM AND CILASTATIN
Route/format = INTRAVENOUS / POWDER
Application Type = A
Applicant Name = ACS DOBFAR
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 250MG BASE/VIAL;250MG/VIAL
----
Product id = 12706
Application Number = 90577
Date of Application = 21,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = IMIPENEM AND CILASTATIN
Route/format = INTRAVENOUS / POWDER
Application Type = A
Applicant Name = ACS DOBFAR
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL;500MG/VIAL
----
Product id = 12707
Application Number = 90825
Date of Application = 16,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = IMIPENEM AND CILASTATIN
Route/format = INTRAVENOUS / POWDER
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 250MG BASE/VIAL;250MG/VIAL
----
Product id = 12708
Application Number = 90825
Date of Application = 16,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = IMIPENEM AND CILASTATIN
Route/format = INTRAVENOUS / POWDER
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL;500MG/VIAL
----
Product id = 12709
Application Number = 91007
Date of Application = 16,, Nov, 2011
RX/OTC/DISCN = RX
Tradename = IMIPENEM AND CILASTATIN
Route/format = INTRAVENOUS / POWDER
Application Type = A
Applicant Name = HOSPIRA INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 500MG BASE/VIAL;500MG/VIAL
----