ciprofloxacin-hydrochloride
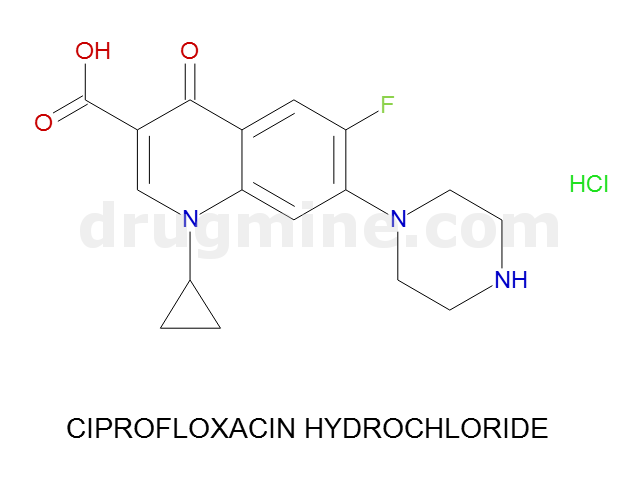

Name: CIPROFLOXACIN HYDROCHLORIDE
ID :
MW: 331
Number of atoms: 24
Molecular_Formula: C17H18FN3O3
Alogp: -1.27
Indication class : Antibacterial
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
ciprofloxacin hydrochloride containing products summary
There are in total 87 different products containing the active ingredient ciprofloxacin hydrochloride. From the 87 drug products, 26 have been discontinued.Product id = 19594
Application Number = 19537
Date of Application = 8,, Apr, 1996
RX/OTC/DISCN = RX
Tradename = CIPRO
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 100MG BASE
----
Product id = 19595
Application Number = 19537
Date of Application = 22,, Oct, 1987
RX/OTC/DISCN = RX
Tradename = CIPRO
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19596
Application Number = 19537
Date of Application = 22,, Oct, 1987
RX/OTC/DISCN = RX
Tradename = CIPRO
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = EQ 500MG BASE
----
Product id = 19597
Application Number = 19537
Date of Application = 22,, Oct, 1987
RX/OTC/DISCN = RX
Tradename = CIPRO
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 750MG BASE
----
Product id = 12902
Application Number = 19992
Date of Application = 31,, Dec, 1990
RX/OTC/DISCN = RX
Tradename = CILOXAN
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = N
Applicant Name = ALCON PHARMS LTD
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = EQ 0.3% BASE
----
Product id = 12321
Application Number = 20369
Date of Application = 30,, Mar, 1998
RX/OTC/DISCN = RX
Tradename = CILOXAN
Route/format = OPHTHALMIC / OINTMENT
Application Type = N
Applicant Name = ALCON PHARMS LTD
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 0.3% BASE
----
Product id = 14594
Application Number = 20805
Date of Application = 10,, Feb, 1998
RX/OTC/DISCN = RX
Tradename = CIPRO HC
Route/format = OTIC / SUSPENSION/DROPS
Application Type = N
Applicant Name = ALCON PHARMS LTD
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 0.2% BASE;1%
----
Product id = 15814
Application Number = 21473
Date of Application = 13,, Dec, 2002
RX/OTC/DISCN = DISCN
Tradename = CIPRO XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = 212.6MG;EQ 287.5MG BASE
----
Product id = 15815
Application Number = 21473
Date of Application = 28,, Aug, 2003
RX/OTC/DISCN = DISCN
Tradename = CIPRO XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = BAYER HLTHCARE
ProductNo = 002
Tecode =
Rld = No
Strength = 425.2MG;EQ 574.9MG BASE
----
Product id = 16550
Application Number = 21744
Date of Application = 19,, May, 2005
RX/OTC/DISCN = DISCN
Tradename = PROQUIN XR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = DEPOMED INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 13217
Application Number = 21918
Date of Application = 1,, May, 2009
RX/OTC/DISCN = RX
Tradename = CETRAXAL
Route/format = OTIC / SOLUTION/DROPS
Application Type = N
Applicant Name = WRASER PHARMS
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 0.2% BASE
----
Product id = 19604
Application Number = 74124
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19605
Application Number = 74124
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 19606
Application Number = 74124
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BARR
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 750MG BASE
----
Product id = 19613
Application Number = 75593
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 750MG BASE
----
Product id = 19610
Application Number = 75593
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 100MG BASE
----
Product id = 19611
Application Number = 75593
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19612
Application Number = 75593
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 19625
Application Number = 75685
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 750MG BASE
----
Product id = 19621
Application Number = 75685
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19623
Application Number = 75685
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 19634
Application Number = 75747
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19635
Application Number = 75747
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 19636
Application Number = 75747
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = RANBAXY
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 750MG BASE
----
Product id = 19620
Application Number = 75817
Date of Application = 25,, Jun, 2007
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 100MG BASE
----
Product id = 19622
Application Number = 75817
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19624
Application Number = 75817
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 19626
Application Number = 75817
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 750MG BASE
----
Product id = 12904
Application Number = 75928
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = APOTEX INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.3% BASE
----
Product id = 19637
Application Number = 75939
Date of Application = 3,, Mar, 2005
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 100MG BASE
----
Product id = 19638
Application Number = 75939
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19640
Application Number = 75939
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 19642
Application Number = 75939
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 750MG BASE
----
Product id = 19617
Application Number = 76089
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19618
Application Number = 76089
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 19619
Application Number = 76089
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = IVAX SUB TEVA PHARMS
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 750MG BASE
----
Product id = 19607
Application Number = 76126
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CARLSBAD
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19608
Application Number = 76126
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CARLSBAD
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 19609
Application Number = 76126
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = CARLSBAD
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 750MG BASE
----
Product id = 19648
Application Number = 76136
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19649
Application Number = 76136
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 19650
Application Number = 76136
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TEVA
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 750MG BASE
----
Product id = 19627
Application Number = 76138
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NOSTRUM LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19628
Application Number = 76138
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NOSTRUM LABS
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 19629
Application Number = 76138
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = NOSTRUM LABS
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 750MG BASE
----
Product id = 19630
Application Number = 76426
Date of Application = 15,, Jun, 2005
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 100MG BASE
----
Product id = 19631
Application Number = 76426
Date of Application = 15,, Jun, 2005
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19632
Application Number = 76426
Date of Application = 15,, Jun, 2005
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 19633
Application Number = 76426
Date of Application = 15,, Jun, 2005
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PLIVA
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 750MG BASE
----
Product id = 12903
Application Number = 76555
Date of Application = 11,, Dec, 2008
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = AKORN INC
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.3% BASE
----
Product id = 19614
Application Number = 76558
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19615
Application Number = 76558
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 19616
Application Number = 76558
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = HIKMA
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 750MG BASE
----
Product id = 19639
Application Number = 76593
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19641
Application Number = 76593
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 19643
Application Number = 76593
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 004
Tecode =
Rld = No
Strength = EQ 750MG BASE
----
Product id = 19651
Application Number = 76639
Date of Application = 10,, Sep, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNIQUE PHARM LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19652
Application Number = 76639
Date of Application = 10,, Sep, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNIQUE PHARM LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 19653
Application Number = 76639
Date of Application = 10,, Sep, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = UNIQUE PHARM LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 750MG BASE
----
Product id = 12909
Application Number = 76673
Date of Application = 21,, Jan, 2005
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.3% BASE
----
Product id = 12906
Application Number = 76754
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = IGI LABS INC
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.3% BASE
----
Product id = 19654
Application Number = 76794
Date of Application = 10,, Feb, 2005
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 100MG BASE
----
Product id = 19655
Application Number = 76794
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19656
Application Number = 76794
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 19657
Application Number = 76794
Date of Application = 9,, Jun, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 750MG BASE
----
Product id = 19598
Application Number = 76896
Date of Application = 4,, Nov, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19599
Application Number = 76896
Date of Application = 4,, Nov, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 19600
Application Number = 76896
Date of Application = 4,, Nov, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = APOTEX
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 750MG BASE
----
Product id = 19644
Application Number = 76912
Date of Application = 18,, Feb, 2005
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 100MG BASE
----
Product id = 19645
Application Number = 76912
Date of Application = 6,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TARO
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19646
Application Number = 76912
Date of Application = 6,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TARO
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 19647
Application Number = 76912
Date of Application = 6,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = TARO
ProductNo = 004
Tecode = AB
Rld = No
Strength = EQ 750MG BASE
----
Product id = 15816
Application Number = 77417
Date of Application = 30,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN EXTENDED RELEASE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 212.6MG;EQ 287.5MG BASE
----
Product id = 12905
Application Number = 77568
Date of Application = 30,, Jun, 2008
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = FDC LTD
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.3% BASE
----
Product id = 12907
Application Number = 77689
Date of Application = 13,, Dec, 2006
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = NEXUS PHARMS
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.3% BASE
----
Product id = 15821
Application Number = 77701
Date of Application = 26,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN EXTENDED RELEASE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 425.2MG;EQ 574.9MG BASE
----
Product id = 15820
Application Number = 77701
Date of Application = 31,, Oct, 2007
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN EXTENDED RELEASE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 002
Tecode =
Rld = No
Strength = 212.6MG;EQ 287.5MG BASE
----
Product id = 15817
Application Number = 77809
Date of Application = 30,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN EXTENDED RELEASE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ACTAVIS LABS FL INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 425.2MG;EQ 574.9MG BASE
----
Product id = 19601
Application Number = 77859
Date of Application = 26,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 250MG BASE
----
Product id = 19602
Application Number = 77859
Date of Application = 26,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 002
Tecode = AB
Rld = No
Strength = EQ 500MG BASE
----
Product id = 19603
Application Number = 77859
Date of Application = 26,, Apr, 2007
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = AUROBINDO PHARMA
ProductNo = 003
Tecode = AB
Rld = No
Strength = EQ 750MG BASE
----
Product id = 15819
Application Number = 78166
Date of Application = 27,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN EXTENDED RELEASE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ANCHEN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 425.2MG;EQ 574.9MG BASE
----
Product id = 15818
Application Number = 78166
Date of Application = 27,, Nov, 2007
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN EXTENDED RELEASE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = ANCHEN PHARMS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 212.6MG;EQ 287.5MG BASE
----
Product id = 15822
Application Number = 78183
Date of Application = 22,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN EXTENDED RELEASE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 212.6MG;EQ 287.5MG BASE
----
Product id = 15823
Application Number = 78183
Date of Application = 22,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN EXTENDED RELEASE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = 425.2MG;EQ 574.9MG BASE
----
Product id = 12908
Application Number = 78598
Date of Application = 16,, Jan, 2008
RX/OTC/DISCN = RX
Tradename = CIPROFLOXACIN HYDROCHLORIDE
Route/format = OPHTHALMIC / SOLUTION/DROPS
Application Type = A
Applicant Name = PHARMAFORCE
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 0.3% BASE
----
Product id = 15824
Application Number = 78712
Date of Application = 11,, Dec, 2007
RX/OTC/DISCN = DISCN
Tradename = CIPROFLOXACIN EXTENDED RELEASE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode =
Rld = No
Strength = 212.6MG;EQ 287.5MG BASE
----