clindamycin-phosphate
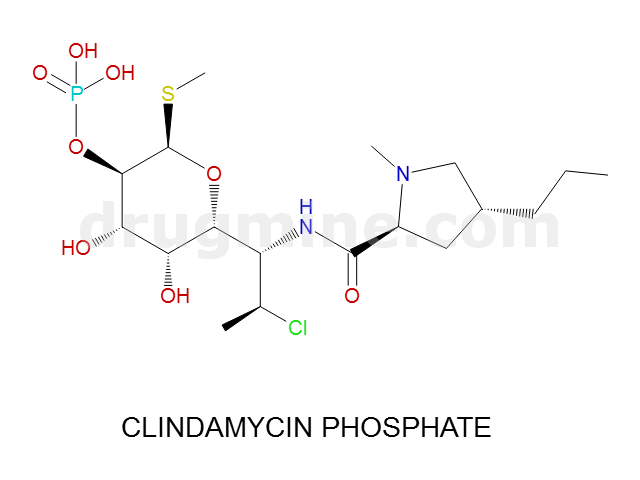
Name: CLINDAMYCIN PHOSPHATE
ID :
MW: 505
Number of atoms: 31
Molecular_Formula: C18H34ClN2O8PS
Alogp: 1.205
Indication class : Antibacterial
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
clindamycin phosphate containing products summary
There are in total 81 different products containing the active ingredient clindamycin phosphate. From the 81 drug products, 29 have been discontinued.Product id = 6748
Application Number = 50441
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CLEOCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 150MG BASE/ML
----
Product id = 14138
Application Number = 50537
Date of Application = Prior, Approved, to
RX/OTC/DISCN = RX
Tradename = CLEOCIN T
Route/format = TOPICAL / SOLUTION
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = EQ 1% BASE
----
Product id = 14859
Application Number = 50537
Date of Application = 22,, Feb, 1994
RX/OTC/DISCN = RX
Tradename = CLEOCIN
Route/format = TOPICAL / SWAB
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 002
Tecode = AT
Rld = No
Strength = EQ 1% BASE
----
Product id = 12172
Application Number = 50600
Date of Application = 31,, May, 1989
RX/OTC/DISCN = RX
Tradename = CLEOCIN T
Route/format = TOPICAL / LOTION
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 1% BASE
----
Product id = 5377
Application Number = 50615
Date of Application = 7,, Jan, 1987
RX/OTC/DISCN = RX
Tradename = CLEOCIN T
Route/format = TOPICAL / GEL
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = EQ 1% BASE
----
Product id = 6783
Application Number = 50635
Date of Application = 22,, Dec, 1989
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE IN DEXTROSE 5%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 900MG BASE/100ML
----
Product id = 6782
Application Number = 50636
Date of Application = 22,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE IN DEXTROSE 5%
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 12MG BASE/ML
----
Product id = 6749
Application Number = 50639
Date of Application = 30,, Aug, 1989
RX/OTC/DISCN = RX
Tradename = CLEOCIN PHOSPHATE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode = AP
Rld = Yes
Strength = EQ 6MG BASE/ML
----
Product id = 6750
Application Number = 50639
Date of Application = 30,, Aug, 1989
RX/OTC/DISCN = RX
Tradename = CLEOCIN PHOSPHATE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 002
Tecode = AP
Rld = Yes
Strength = EQ 12MG BASE/ML
----
Product id = 6751
Application Number = 50639
Date of Application = 10,, Apr, 1991
RX/OTC/DISCN = RX
Tradename = CLEOCIN PHOSPHATE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 003
Tecode = AP
Rld = Yes
Strength = EQ 18MG BASE/ML
----
Product id = 6787
Application Number = 50648
Date of Application = 29,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 6MG BASE/ML
----
Product id = 6788
Application Number = 50648
Date of Application = 29,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 12MG BASE/ML
----
Product id = 6789
Application Number = 50648
Date of Application = 29,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = N
Applicant Name = BAXTER HLTHCARE
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 900MG BASE/100ML
----
Product id = 4468
Application Number = 50680
Date of Application = 11,, Aug, 1992
RX/OTC/DISCN = DISCN
Tradename = CLEOCIN
Route/format = VAGINAL / CREAM
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 2% BASE
----
Product id = 4469
Application Number = 50680
Date of Application = 2,, Mar, 1998
RX/OTC/DISCN = RX
Tradename = CLEOCIN
Route/format = VAGINAL / CREAM
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 002
Tecode = AB
Rld = Yes
Strength = EQ 2% BASE
----
Product id = 5393
Application Number = 50741
Date of Application = 26,, Aug, 2002
RX/OTC/DISCN = RX
Tradename = DUAC
Route/format = TOPICAL / GEL
Application Type = N
Applicant Name = STIEFEL
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 5%;1.2%
----
Product id = 5370
Application Number = 50756
Date of Application = 21,, Dec, 2000
RX/OTC/DISCN = RX
Tradename = BENZACLIN
Route/format = TOPICAL / GEL
Application Type = N
Applicant Name = VALEANT BERMUDA
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 5%;EQ 1% BASE
----
Product id = 5371
Application Number = 50756
Date of Application = 20,, Apr, 2007
RX/OTC/DISCN = DISCN
Tradename = BENZACLIN
Route/format = TOPICAL / GEL
Application Type = N
Applicant Name = VALEANT BERMUDA
ProductNo = 002
Tecode =
Rld = No
Strength = 5%;EQ 1% BASE
----
Product id = 14499
Application Number = 50767
Date of Application = 13,, Aug, 1999
RX/OTC/DISCN = RX
Tradename = CLEOCIN
Route/format = VAGINAL / SUPPOSITORY
Application Type = N
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode =
Rld = Yes
Strength = 100MG
----
Product id = 5378
Application Number = 50782
Date of Application = 27,, Nov, 2000
RX/OTC/DISCN = RX
Tradename = CLINDAGEL
Route/format = TOPICAL / GEL
Application Type = N
Applicant Name = PRECISION DERMAT
ProductNo = 001
Tecode = BT
Rld = Yes
Strength = EQ 1% BASE
----
Product id = 4471
Application Number = 50793
Date of Application = 30,, Nov, 2004
RX/OTC/DISCN = RX
Tradename = CLINDESSE
Route/format = VAGINAL / CREAM
Application Type = N
Applicant Name = ELAN PHARMA INTL LTD
ProductNo = 001
Tecode =
Rld = Yes
Strength = EQ 2% BASE
----
Product id = 7
Application Number = 50801
Date of Application = 22,, Oct, 2004
RX/OTC/DISCN = RX
Tradename = EVOCLIN
Route/format = TOPICAL / AEROSOL, FOAM
Application Type = N
Applicant Name = DELCOR ASSET
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 1%
----
Product id = 5449
Application Number = 50802
Date of Application = 7,, Nov, 2006
RX/OTC/DISCN = RX
Tradename = ZIANA
Route/format = TOPICAL / GEL
Application Type = N
Applicant Name = MEDICIS
ProductNo = 001
Tecode = BX
Rld = Yes
Strength = 1.2%;0.025%
----
Product id = 5446
Application Number = 50803
Date of Application = 16,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = VELTIN
Route/format = TOPICAL / GEL
Application Type = N
Applicant Name = STIEFEL GSK
ProductNo = 001
Tecode = BX
Rld = Yes
Strength = 1.2%;0.025%
----
Product id = 5361
Application Number = 50819
Date of Application = 23,, Oct, 2008
RX/OTC/DISCN = RX
Tradename = ACANYA
Route/format = TOPICAL / GEL
Application Type = N
Applicant Name = DOW PHARM
ProductNo = 001
Tecode =
Rld = Yes
Strength = 2.5%;EQ 1.2% BASE
----
Product id = 5423
Application Number = 50819
Date of Application = 24,, Nov, 2014
RX/OTC/DISCN = RX
Tradename = ONEXTON
Route/format = TOPICAL / GEL
Application Type = N
Applicant Name = DOW PHARM
ProductNo = 002
Tecode =
Rld = Yes
Strength = 3.75%;EQ 1.2% BASE
----
Product id = 6746
Application Number = 61839
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CLEOCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 14137
Application Number = 62363
Date of Application = 8,, Feb, 1982
RX/OTC/DISCN = DISCN
Tradename = CLEOCIN T
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1% BASE
----
Product id = 6752
Application Number = 62747
Date of Application = 3,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABRAXIS PHARM
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 6763
Application Number = 62800
Date of Application = 24,, Jul, 1987
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 6764
Application Number = 62801
Date of Application = 24,, Jul, 1987
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 6747
Application Number = 62803
Date of Application = 16,, Oct, 1987
RX/OTC/DISCN = RX
Tradename = CLEOCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = PHARMACIA AND UPJOHN
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 6759
Application Number = 62806
Date of Application = 15,, Oct, 1987
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 14143
Application Number = 62811
Date of Application = 1,, Sep, 1988
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = G AND W LABS INC
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 1% BASE
----
Product id = 6770
Application Number = 62819
Date of Application = 15,, Mar, 1988
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SOLOPAK
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 6771
Application Number = 62852
Date of Application = 17,, Mar, 1988
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SOLOPAK
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 6760
Application Number = 62889
Date of Application = 25,, Apr, 1988
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 6774
Application Number = 62900
Date of Application = 8,, Jun, 1988
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 6766
Application Number = 62905
Date of Application = 9,, May, 1988
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = LOCH
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 6756
Application Number = 62908
Date of Application = 1,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BRISTOL MYERS SQUIBB
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 6767
Application Number = 62913
Date of Application = 20,, Oct, 1988
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = MARSAM PHARMS LLC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 6753
Application Number = 62928
Date of Application = 13,, Feb, 1989
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ASTRAZENECA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 14147
Application Number = 62930
Date of Application = 28,, Jun, 1989
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = VINTAGE PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1% BASE
----
Product id = 6765
Application Number = 62943
Date of Application = 29,, Sep, 1988
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HOSPIRA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 14140
Application Number = 62944
Date of Application = 11,, Jan, 1989
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = BOCA PHARMA LLC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1% BASE
----
Product id = 6761
Application Number = 62953
Date of Application = 21,, Apr, 1988
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 6772
Application Number = 63041
Date of Application = 29,, Dec, 1989
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PARENTERAL
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 6762
Application Number = 63068
Date of Application = 28,, Aug, 1989
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = HIKMA MAPLE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 6775
Application Number = 63079
Date of Application = 5,, Mar, 1990
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = WATSON LABS
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 6754
Application Number = 63163
Date of Application = 30,, Jun, 1994
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 6773
Application Number = 63282
Date of Application = 29,, May, 1992
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = TEVA PARENTERAL
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 14148
Application Number = 63304
Date of Application = 15,, Jul, 1997
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 1% BASE
----
Product id = 14139
Application Number = 63329
Date of Application = 30,, Sep, 1992
RX/OTC/DISCN = RX
Tradename = CLINDA-DERM
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = PADDOCK LLC
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 1% BASE
----
Product id = 14144
Application Number = 64050
Date of Application = 30,, Nov, 1995
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 1% BASE
----
Product id = 14145
Application Number = 64108
Date of Application = 27,, Sep, 1996
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = RENAISSANCE PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 1% BASE
----
Product id = 14862
Application Number = 64136
Date of Application = 30,, Sep, 1996
RX/OTC/DISCN = RX
Tradename = CLINDETS
Route/format = TOPICAL / SWAB
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 1% BASE
----
Product id = 14141
Application Number = 64159
Date of Application = 5,, Jun, 1997
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = FOUGERA
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 1% BASE
----
Product id = 5379
Application Number = 64160
Date of Application = 28,, Jan, 2000
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = TOPICAL / GEL
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1% BASE
----
Product id = 6784
Application Number = 65027
Date of Application = 29,, Jun, 2001
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABBVIE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 6MG BASE/ML
----
Product id = 6785
Application Number = 65027
Date of Application = 29,, Jun, 2001
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABBVIE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 12MG BASE/ML
----
Product id = 6786
Application Number = 65027
Date of Application = 29,, Jun, 2001
RX/OTC/DISCN = DISCN
Tradename = CLINDAMYCIN PHOSPHATE IN DEXTROSE 5% IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = ABBVIE
ProductNo = 003
Tecode =
Rld = No
Strength = EQ 18MG BASE/ML
----
Product id = 14860
Application Number = 65049
Date of Application = 25,, May, 2000
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = TOPICAL / SWAB
Application Type = A
Applicant Name = PERRIGO NEW YORK
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 1% BASE
----
Product id = 12173
Application Number = 65067
Date of Application = 31,, Jan, 2002
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 1% BASE
----
Product id = 4470
Application Number = 65139
Date of Application = 27,, Dec, 2004
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = VAGINAL / CREAM
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = EQ 2% BASE
----
Product id = 14146
Application Number = 65184
Date of Application = 31,, Mar, 2004
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = TARO PHARM INDS
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 1% BASE
----
Product id = 6755
Application Number = 65206
Date of Application = 24,, Sep, 2004
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = BEDFORD
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 14142
Application Number = 65254
Date of Application = 14,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 1% BASE
----
Product id = 6757
Application Number = 65346
Date of Application = 29,, Mar, 2007
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 6758
Application Number = 65347
Date of Application = 9,, May, 2007
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = FRESENIUS KABI USA
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 5380
Application Number = 65443
Date of Application = 11,, Aug, 2009
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE AND BENZOYL PEROXIDE
Route/format = TOPICAL / GEL
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5%;EQ 1% BASE
----
Product id = 14861
Application Number = 65513
Date of Application = 17,, Jun, 2010
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = TOPICAL / SWAB
Application Type = A
Applicant Name = VERSAPHARM
ProductNo = 001
Tecode = AT
Rld = No
Strength = EQ 1% BASE
----
Product id = 6768
Application Number = 90108
Date of Application = 30,, Sep, 2011
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT PHARMS
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 6769
Application Number = 90109
Date of Application = 30,, Sep, 2011
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SAGENT STRIDES
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 150MG BASE/ML
----
Product id = 3
Application Number = 90785
Date of Application = 31,, Mar, 2010
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE
Route/format = TOPICAL / AEROSOL, FOAM
Application Type = A
Applicant Name = PERRIGO UK FINCO
ProductNo = 001
Tecode = AT
Rld = No
Strength = 1%
----
Product id = 5381
Application Number = 90979
Date of Application = 26,, Jun, 2012
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE AND BENZOYL PEROXIDE
Route/format = TOPICAL / GEL
Application Type = A
Applicant Name = PERRIGO ISRAEL
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5%;1.2%
----
Product id = 6779
Application Number = 201692
Date of Application = 31,, May, 2012
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE IN 5% DEXTROSE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 6MG BASE/ML
----
Product id = 6780
Application Number = 201692
Date of Application = 31,, May, 2012
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE IN 5% DEXTROSE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 12MG BASE/ML
----
Product id = 6781
Application Number = 201692
Date of Application = 31,, May, 2012
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE IN 5% DEXTROSE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = SANDOZ INC
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 18MG BASE/ML
----
Product id = 6776
Application Number = 203048
Date of Application = 4,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE IN 5% DEXTROSE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AKORN INC
ProductNo = 001
Tecode = AP
Rld = No
Strength = EQ 6MG BASE/ML
----
Product id = 6777
Application Number = 203048
Date of Application = 4,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE IN 5% DEXTROSE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AKORN INC
ProductNo = 002
Tecode = AP
Rld = No
Strength = EQ 12MG BASE/ML
----
Product id = 6778
Application Number = 203048
Date of Application = 4,, Apr, 2013
RX/OTC/DISCN = RX
Tradename = CLINDAMYCIN PHOSPHATE IN 5% DEXTROSE IN PLASTIC CONTAINER
Route/format = INJECTION / INJECTABLE
Application Type = A
Applicant Name = AKORN INC
ProductNo = 003
Tecode = AP
Rld = No
Strength = EQ 18MG BASE/ML
----