clobetasol-propionate
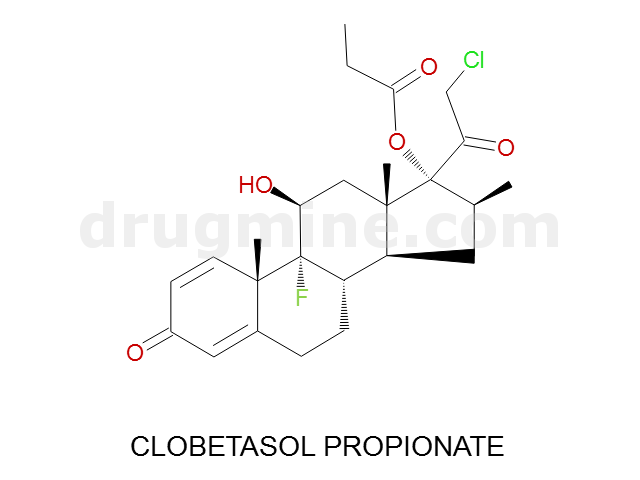

Name: CLOBETASOL PROPIONATE
ID :
MW: 467
Number of atoms: 32
Molecular_Formula: C25H32ClFO5
Alogp: 3.958
Indication class : Anti-Inflammatory
Oral Flag : 0
Max_Phase : 4
Molecule_Type : Small molecule
clobetasol propionate containing products summary
There are in total 44 different products containing the active ingredient clobetasol propionate. From the 44 drug products, 7 have been discontinued.Product id = 4391
Application Number = 19322
Date of Application = 27,, Dec, 1985
RX/OTC/DISCN = RX
Tradename = TEMOVATE
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB1
Rld = Yes
Strength = 0.05%
----
Product id = 12579
Application Number = 19323
Date of Application = 27,, Dec, 1985
RX/OTC/DISCN = RX
Tradename = TEMOVATE
Route/format = TOPICAL / OINTMENT
Application Type = N
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.05%
----
Product id = 14282
Application Number = 19966
Date of Application = 22,, Feb, 1990
RX/OTC/DISCN = DISCN
Tradename = TEMOVATE
Route/format = TOPICAL / SOLUTION
Application Type = N
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode =
Rld = No
Strength = 0.05% **Federal Register determination that product was not discontinued or withdrawn for safety or efficacy reasons**
----
Product id = 5437
Application Number = 20337
Date of Application = 29,, Apr, 1994
RX/OTC/DISCN = RX
Tradename = TEMOVATE
Route/format = TOPICAL / GEL
Application Type = N
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.05%
----
Product id = 4392
Application Number = 20340
Date of Application = 17,, Jun, 1994
RX/OTC/DISCN = RX
Tradename = TEMOVATE E
Route/format = TOPICAL / CREAM
Application Type = N
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB2
Rld = Yes
Strength = 0.05%
----
Product id = 14
Application Number = 21142
Date of Application = 26,, May, 2000
RX/OTC/DISCN = RX
Tradename = OLUX
Route/format = TOPICAL / AEROSOL, FOAM
Application Type = N
Applicant Name = DELCOR ASSET
ProductNo = 001
Tecode = AB1
Rld = Yes
Strength = 0.05%
----
Product id = 12176
Application Number = 21535
Date of Application = 24,, Jul, 2003
RX/OTC/DISCN = RX
Tradename = CLOBEX
Route/format = TOPICAL / LOTION
Application Type = N
Applicant Name = GALDERMA LABS LP
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.05%
----
Product id = 12817
Application Number = 21644
Date of Application = 5,, Feb, 2004
RX/OTC/DISCN = RX
Tradename = CLOBEX
Route/format = TOPICAL / SHAMPOO
Application Type = N
Applicant Name = GALDERMA LABS
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 0.05%
----
Product id = 14421
Application Number = 21835
Date of Application = 27,, Oct, 2005
RX/OTC/DISCN = RX
Tradename = CLOBEX
Route/format = TOPICAL / SPRAY
Application Type = N
Applicant Name = GALDERMA LABS LP
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.05%
----
Product id = 15
Application Number = 22013
Date of Application = 12,, Jan, 2007
RX/OTC/DISCN = RX
Tradename = OLUX E
Route/format = TOPICAL / AEROSOL, FOAM
Application Type = N
Applicant Name = DELCOR ASSET
ProductNo = 001
Tecode = AB2
Rld = Yes
Strength = 0.05%
----
Product id = 4088
Application Number = 74087
Date of Application = 16,, Feb, 1994
RX/OTC/DISCN = DISCN
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TEVA PHARMS USA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.05%
----
Product id = 12427
Application Number = 74089
Date of Application = 16,, Feb, 1994
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = G AND W LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----
Product id = 12425
Application Number = 74128
Date of Application = 3,, Aug, 1994
RX/OTC/DISCN = DISCN
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode =
Rld = No
Strength = 0.05%
----
Product id = 4085
Application Number = 74139
Date of Application = 3,, Aug, 1994
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = G AND W LABS INC
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 0.05%
----
Product id = 4102
Application Number = 74220
Date of Application = 16,, May, 1997
RX/OTC/DISCN = RX
Tradename = CORMAX
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = HI TECH PHARMA
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 0.05%
----
Product id = 12455
Application Number = 74221
Date of Application = 31,, Mar, 1995
RX/OTC/DISCN = RX
Tradename = EMBELINE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = HI TECH PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----
Product id = 14172
Application Number = 74222
Date of Application = 6,, Dec, 1995
RX/OTC/DISCN = RX
Tradename = EMBELINE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = HI TECH PHARMA
ProductNo = 001
Tecode = AT
Rld = Yes
Strength = 0.05%
----
Product id = 12429
Application Number = 74248
Date of Application = 12,, Jul, 1996
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----
Product id = 4087
Application Number = 74249
Date of Application = 8,, Jul, 1996
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 0.05%
----
Product id = 14150
Application Number = 74331
Date of Application = 15,, Dec, 1995
RX/OTC/DISCN = DISCN
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = G AND W LABS INC
ProductNo = 001
Tecode =
Rld = No
Strength = 0.05%
----
Product id = 4084
Application Number = 74392
Date of Application = 30,, Sep, 1996
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = FOUGERA
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 0.05%
----
Product id = 12426
Application Number = 74407
Date of Application = 23,, Feb, 1996
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----
Product id = 5383
Application Number = 75027
Date of Application = 31,, Oct, 1997
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / GEL
Application Type = A
Applicant Name = PERRIGO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----
Product id = 12428
Application Number = 75057
Date of Application = 12,, Aug, 1998
RX/OTC/DISCN = DISCN
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / OINTMENT
Application Type = A
Applicant Name = RENAISSANCE PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.05%
----
Product id = 14154
Application Number = 75205
Date of Application = 13,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = WOCKHARDT
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.05%
----
Product id = 14151
Application Number = 75224
Date of Application = 16,, Nov, 1998
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.05%
----
Product id = 5384
Application Number = 75279
Date of Application = 28,, May, 1999
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / GEL
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----
Product id = 4137
Application Number = 75325
Date of Application = 24,, Dec, 1998
RX/OTC/DISCN = RX
Tradename = EMBELINE E
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = HI TECH PHARMA
ProductNo = 001
Tecode = AB2
Rld = No
Strength = 0.05%
----
Product id = 4086
Application Number = 75338
Date of Application = 9,, Feb, 2001
RX/OTC/DISCN = DISCN
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = RENAISSANCE PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.05%
----
Product id = 14152
Application Number = 75363
Date of Application = 29,, Dec, 2000
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.05%
----
Product id = 5382
Application Number = 75368
Date of Application = 15,, Feb, 2000
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / GEL
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----
Product id = 14149
Application Number = 75391
Date of Application = 8,, Feb, 1999
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.05%
----
Product id = 4089
Application Number = 75430
Date of Application = 26,, May, 1999
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE (EMOLLIENT)
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = FOUGERA PHARMS
ProductNo = 001
Tecode = AB2
Rld = No
Strength = 0.05%
----
Product id = 4091
Application Number = 75633
Date of Application = 17,, May, 2000
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE (EMOLLIENT)
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB2
Rld = No
Strength = 0.05%
----
Product id = 4090
Application Number = 75733
Date of Application = 22,, Aug, 2001
RX/OTC/DISCN = DISCN
Tradename = CLOBETASOL PROPIONATE (EMOLLIENT)
Route/format = TOPICAL / CREAM
Application Type = A
Applicant Name = RENAISSANCE PHARMA
ProductNo = 001
Tecode =
Rld = No
Strength = 0.05%
----
Product id = 5395
Application Number = 76141
Date of Application = 12,, Apr, 2002
RX/OTC/DISCN = RX
Tradename = EMBELINE
Route/format = TOPICAL / GEL
Application Type = A
Applicant Name = HI TECH PHARMA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----
Product id = 14153
Application Number = 76977
Date of Application = 5,, Aug, 2005
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / SOLUTION
Application Type = A
Applicant Name = TOLMAR
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.05%
----
Product id = 4
Application Number = 77763
Date of Application = 10,, Mar, 2008
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / AEROSOL, FOAM
Application Type = A
Applicant Name = PERRIGO ISRAEL
ProductNo = 001
Tecode = AB1
Rld = No
Strength = 0.05%
----
Product id = 12174
Application Number = 78223
Date of Application = 4,, Dec, 2008
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----
Product id = 12815
Application Number = 78854
Date of Application = 7,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / SHAMPOO
Application Type = A
Applicant Name = ACTAVIS MID ATLANTIC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----
Product id = 14420
Application Number = 90898
Date of Application = 16,, Jun, 2011
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / SPRAY
Application Type = A
Applicant Name = PADDOCK LLC
ProductNo = 001
Tecode = AT
Rld = No
Strength = 0.05%
----
Product id = 12816
Application Number = 90974
Date of Application = 9,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / SHAMPOO
Application Type = A
Applicant Name = PERRIGO ISRAEL
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----
Product id = 12175
Application Number = 200302
Date of Application = 2,, Jul, 2012
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / LOTION
Application Type = A
Applicant Name = TARO
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.05%
----
Product id = 5
Application Number = 201402
Date of Application = 14,, Aug, 2012
RX/OTC/DISCN = RX
Tradename = CLOBETASOL PROPIONATE
Route/format = TOPICAL / AEROSOL, FOAM
Application Type = A
Applicant Name = PERRIGO ISRAEL
ProductNo = 001
Tecode = AB2
Rld = No
Strength = 0.05%
----