clonidine
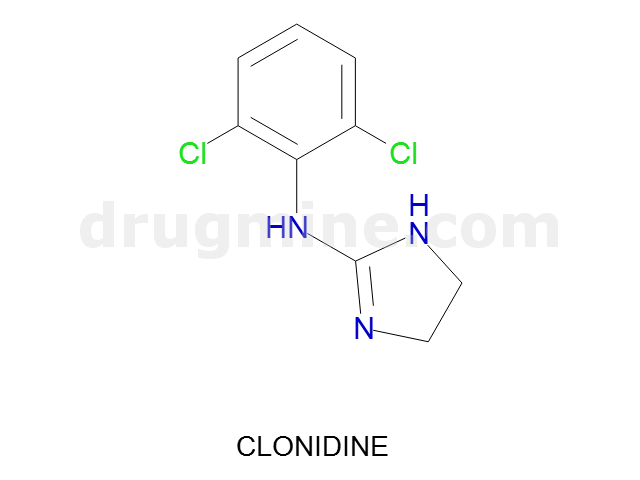

Name: CLONIDINE
ID :
MW: 230
Number of atoms: 14
Molecular_Formula: C9H9Cl2N3
Alogp: 2.354
Indication class : Antihypertensive
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
clonidine containing products summary
There are in total 18 different products containing the active ingredient clonidine. From the 18 drug products, 3 have been discontinued.Product id = 4624
Application Number = 18891
Date of Application = 10,, Oct, 1984
RX/OTC/DISCN = RX
Tradename = CATAPRES-TTS-1
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.1MG/24HR
----
Product id = 4625
Application Number = 18891
Date of Application = 10,, Oct, 1984
RX/OTC/DISCN = RX
Tradename = CATAPRES-TTS-2
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.2MG/24HR
----
Product id = 4626
Application Number = 18891
Date of Application = 10,, Oct, 1984
RX/OTC/DISCN = RX
Tradename = CATAPRES-TTS-3
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = N
Applicant Name = BOEHRINGER INGELHEIM
ProductNo = 003
Tecode = AB
Rld = Yes
Strength = 0.3MG/24HR
----
Product id = 14527
Application Number = 22499
Date of Application = 3,, Dec, 2009
RX/OTC/DISCN = DISCN
Tradename = CLONIDINE
Route/format = ORAL / SUSPENSION, EXTENDED RELEASE
Application Type = N
Applicant Name = TRIS PHARMA INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.09MG BASE/ML
----
Product id = 15833
Application Number = 22500
Date of Application = 3,, Dec, 2009
RX/OTC/DISCN = DISCN
Tradename = CLONIDINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = TRIS PHARMA INC
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 0.17MG BASE
----
Product id = 15834
Application Number = 22500
Date of Application = 3,, Dec, 2009
RX/OTC/DISCN = DISCN
Tradename = CLONIDINE
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = TRIS PHARMA INC
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 0.26MG BASE
----
Product id = 4634
Application Number = 76157
Date of Application = 18,, Aug, 2009
RX/OTC/DISCN = RX
Tradename = CLONIDINE
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = AVEVA
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.1MG/24HR
----
Product id = 4635
Application Number = 76157
Date of Application = 18,, Aug, 2009
RX/OTC/DISCN = RX
Tradename = CLONIDINE
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = AVEVA
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.2MG/24HR
----
Product id = 4636
Application Number = 76157
Date of Application = 18,, Aug, 2009
RX/OTC/DISCN = RX
Tradename = CLONIDINE
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = AVEVA
ProductNo = 003
Tecode = AB
Rld = No
Strength = 0.3MG/24HR
----
Product id = 4640
Application Number = 76166
Date of Application = 16,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = CLONIDINE
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN TECHNOLOGIES
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.1MG/24HR
----
Product id = 4641
Application Number = 76166
Date of Application = 16,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = CLONIDINE
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN TECHNOLOGIES
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.2MG/24HR
----
Product id = 4642
Application Number = 76166
Date of Application = 16,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = CLONIDINE
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = MYLAN TECHNOLOGIES
ProductNo = 003
Tecode = AB
Rld = No
Strength = 0.3MG/24HR
----
Product id = 4637
Application Number = 79090
Date of Application = 20,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = CLONIDINE
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = BARR
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.1MG/24HR
----
Product id = 4638
Application Number = 79090
Date of Application = 20,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = CLONIDINE
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = BARR
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.2MG/24HR
----
Product id = 4639
Application Number = 79090
Date of Application = 20,, Aug, 2010
RX/OTC/DISCN = RX
Tradename = CLONIDINE
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = BARR
ProductNo = 003
Tecode = AB
Rld = No
Strength = 0.3MG/24HR
----
Product id = 4643
Application Number = 90873
Date of Application = 6,, May, 2014
RX/OTC/DISCN = RX
Tradename = CLONIDINE
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 0.1MG/24HR
----
Product id = 4644
Application Number = 90873
Date of Application = 6,, May, 2014
RX/OTC/DISCN = RX
Tradename = CLONIDINE
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 002
Tecode = AB
Rld = No
Strength = 0.2MG/24HR
----
Product id = 4645
Application Number = 90873
Date of Application = 6,, May, 2014
RX/OTC/DISCN = RX
Tradename = CLONIDINE
Route/format = TRANSDERMAL / FILM, EXTENDED RELEASE
Application Type = A
Applicant Name = WATSON LABS INC
ProductNo = 003
Tecode = AB
Rld = No
Strength = 0.3MG/24HR
----