cloxacillin-sodium
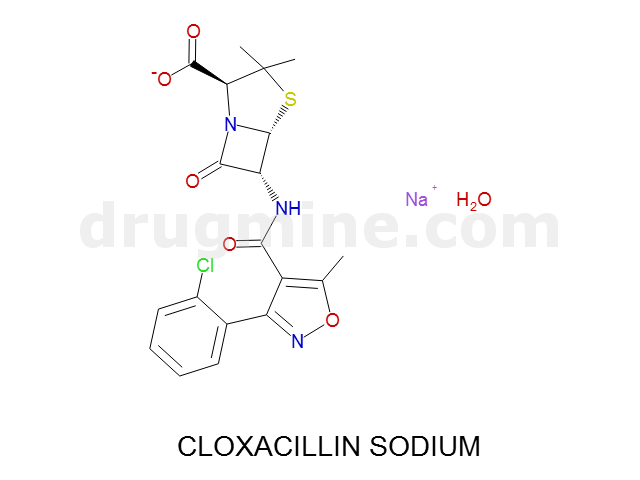

Name: CLOXACILLIN SODIUM
ID :
MW: 435
Number of atoms: 29
Molecular_Formula: C19H17ClN3O5S
Alogp: 0.826
Indication class : Antibacterial
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
cloxacillin sodium containing products summary
There are in total 12 different products containing the active ingredient cloxacillin sodium. From the 12 drug products, 12 have been discontinued.Product id = 4935
Application Number = 50192
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TEGOPEN
Route/format = ORAL / FOR SOLUTION
Application Type = N
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 125MG BASE/5ML
----
Product id = 1506
Application Number = 61452
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CLOXACILLIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE
----
Product id = 1507
Application Number = 61452
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CLOXACILLIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = APOTHECON
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 4934
Application Number = 61453
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = TEGOPEN
Route/format = ORAL / FOR SOLUTION
Application Type = A
Applicant Name = APOTHECON
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 125MG BASE/5ML
----
Product id = 1510
Application Number = 61806
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CLOXAPEN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE
----
Product id = 1512
Application Number = 61806
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CLOXAPEN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 1511
Application Number = 62233
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CLOXAPEN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE
----
Product id = 1513
Application Number = 62233
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CLOXAPEN
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = GLAXOSMITHKLINE
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 1508
Application Number = 62240
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CLOXACILLIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 250MG BASE
----
Product id = 1509
Application Number = 62240
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CLOXACILLIN SODIUM
Route/format = ORAL / CAPSULE
Application Type = A
Applicant Name = TEVA
ProductNo = 002
Tecode =
Rld = No
Strength = EQ 500MG BASE
----
Product id = 4844
Application Number = 62268
Date of Application = Prior, Approved, to
RX/OTC/DISCN = DISCN
Tradename = CLOXACILLIN SODIUM
Route/format = ORAL / FOR SOLUTION
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 125MG BASE/5ML
----
Product id = 4845
Application Number = 62978
Date of Application = 6,, Apr, 1989
RX/OTC/DISCN = DISCN
Tradename = CLOXACILLIN SODIUM
Route/format = ORAL / FOR SOLUTION
Application Type = A
Applicant Name = TEVA
ProductNo = 001
Tecode =
Rld = No
Strength = EQ 125MG BASE/5ML
----