desloratadine
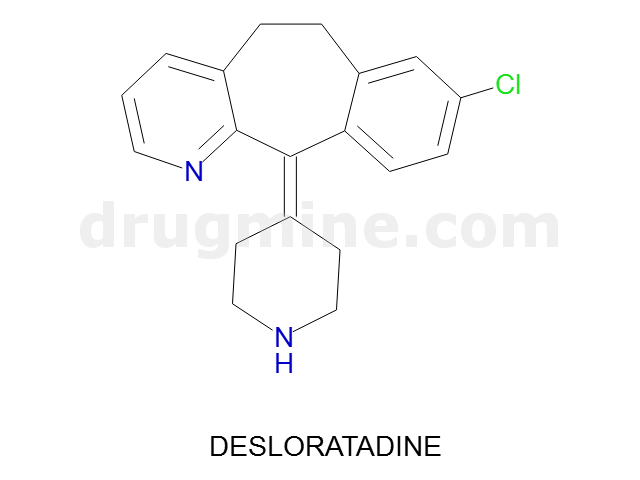


Name: DESLORATADINE
ID :
MW: 311
Number of atoms: 22
Molecular_Formula: C19H19ClN2
Alogp: 4.213
Indication class : .
Oral Flag : 1
Max_Phase : 4
Molecule_Type : Small molecule
desloratadine containing products summary
There are in total 17 different products containing the active ingredient desloratadine. From the 17 drug products, zero have been discontinued.Product id = 19732
Application Number = 21165
Date of Application = 21,, Dec, 2001
RX/OTC/DISCN = RX
Tradename = CLARINEX
Route/format = ORAL / TABLET
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 5MG
----
Product id = 13779
Application Number = 21300
Date of Application = 1,, Sep, 2004
RX/OTC/DISCN = RX
Tradename = CLARINEX
Route/format = ORAL / SOLUTION
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode =
Rld = Yes
Strength = 0.5MG/ML
----
Product id = 16869
Application Number = 21312
Date of Application = 26,, Jun, 2002
RX/OTC/DISCN = RX
Tradename = CLARINEX
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 5MG
----
Product id = 16868
Application Number = 21312
Date of Application = 14,, Jul, 2005
RX/OTC/DISCN = RX
Tradename = CLARINEX
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 002
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 15826
Application Number = 21313
Date of Application = 1,, Feb, 2006
RX/OTC/DISCN = RX
Tradename = CLARINEX-D 12 HOUR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode =
Rld = Yes
Strength = 2.5MG;120MG
----
Product id = 15825
Application Number = 21605
Date of Application = 3,, Mar, 2005
RX/OTC/DISCN = RX
Tradename = CLARINEX D 24 HOUR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = N
Applicant Name = MERCK SHARP DOHME
ProductNo = 001
Tecode = AB
Rld = Yes
Strength = 5MG;240MG
----
Product id = 20194
Application Number = 78351
Date of Application = 10,, Feb, 2012
RX/OTC/DISCN = RX
Tradename = DESLORATADINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = MYLAN PHARMS INC
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 20193
Application Number = 78352
Date of Application = 25,, Oct, 2010
RX/OTC/DISCN = RX
Tradename = DESLORATADINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = LUPIN PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 20191
Application Number = 78355
Date of Application = 19,, Apr, 2012
RX/OTC/DISCN = RX
Tradename = DESLORATADINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = BELCHER PHARMS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 20195
Application Number = 78357
Date of Application = 19,, Feb, 2010
RX/OTC/DISCN = RX
Tradename = DESLORATADINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = ORCHID HLTHCARE
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 20198
Application Number = 78359
Date of Application = 16,, Nov, 2010
RX/OTC/DISCN = RX
Tradename = DESLORATADINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SUN PHARM INDS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 20196
Application Number = 78361
Date of Application = 22,, Dec, 2011
RX/OTC/DISCN = RX
Tradename = DESLORATADINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = PERRIGO R AND D
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 20197
Application Number = 78364
Date of Application = 3,, Dec, 2010
RX/OTC/DISCN = RX
Tradename = DESLORATADINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = SANDOZ
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 20192
Application Number = 78365
Date of Application = 8,, Mar, 2011
RX/OTC/DISCN = RX
Tradename = DESLORATADINE
Route/format = ORAL / TABLET
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG
----
Product id = 15851
Application Number = 78366
Date of Application = 26,, Apr, 2011
RX/OTC/DISCN = RX
Tradename = DESLORATADINE AND PSEUDOEPHEDRINE SULFATE 24 HOUR
Route/format = ORAL / TABLET, EXTENDED RELEASE
Application Type = A
Applicant Name = DR REDDYS LABS LTD
ProductNo = 001
Tecode = AB
Rld = No
Strength = 5MG;240MG
----
Product id = 16888
Application Number = 78367
Date of Application = 12,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = DESLORATADINE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = REDDYS
ProductNo = 001
Tecode = AB
Rld = No
Strength = 2.5MG
----
Product id = 16889
Application Number = 78367
Date of Application = 12,, Jul, 2010
RX/OTC/DISCN = RX
Tradename = DESLORATADINE
Route/format = ORAL / TABLET, ORALLY DISINTEGRATING
Application Type = A
Applicant Name = REDDYS
ProductNo = 002
Tecode = AB
Rld = No
Strength = 5MG
----